Ligelizumab could top Xolair in chronic hives, says Novartis

Novartis’ immunoglobulin E inhibitor Xolair has been a mainstay of treatment for chronic spontaneous urticaria (CSU) for many years, but the company says it has gone one better with new candidate ligelizumab.
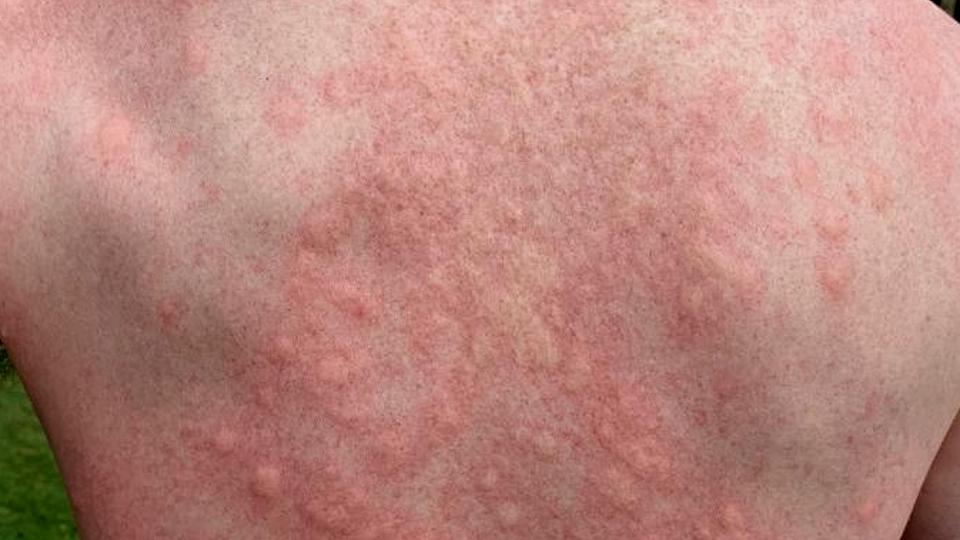
The new-generation drug – also known as QGE031 – binds more tightly to IgE than Roche-partnered Xolair (omalizumab), and that suggests it could be more effective as a treatment for CSU, a distressing and debilitating condition which causes welts (commonly known as ‘hives’) to spring up on the skin.
Like Xolair, ligelizumab works by blocking IgE from binding to mast cells and basophils and causing them to release histamine.
The new data published in the journal Nature Medicine show ligelizumab and Xolair recognise and bind differently to IgE, with ligelizumab showing “significantly enhanced blockade” of IgE pathway signalling.
Xolair has been approved for CSU since 2014, when its launch transformed treatment for patients at the severe end of the symptom spectrum.
Overall, CSU affects around 1% of the global population and in severe cases causes itching and swelling that can last for weeks at a time and resist even very high doses of antihistamines.
The drug is maturing fast however, having been launched in the early 2000s as a treatment for severe allergic asthma associated with IgE. Sales are split between Roche and Novartis and the two companies booked $1.49 billion and $870 million respectively from the drug in the first nine months of 2019.
Ligelizumab has previously shown in a phase 2b trial that it is more effective at keeping CSU patients symptom-free than Xolair, so it could provide a way for Novartis to defend its franchise now that Xolair has lost patent protection in the US and Europe.
Biosimilars haven’t been approved yet but are in development at the likes of Glenmark, Fountain Biopharma, Celltrion and Sorrento/Mabtech.
Ligelizumab is in a phase III trials programme – including the PEARL 1 and PEARL 2 studies – that are recruiting more than 2,000 patients across 48 countries around the world whose CSU is inadequately controlled with antihistamines.
According to clinicaltrials.gov, data from these could be available later this year with study completion dates in 2021.
If approved, ligelizumab could switch Roche and Novartis from allies to enemies in the CSU market. Roche has no interest in the IgE antibody but is developing its own new CSU therapy, an orally-administered BTK inhibitor called fenebrutinib that has reached the phase II trial stage.
“This mechanistic study further supports those findings as we look to reimagine care to bring better treatment options for patients with CSU,” commented Eric Hughes, Novartis’ global development unit head for immunology, hepatology and dermatology.