J&J shows its confidence in oral IL-23 drug for psoriasis
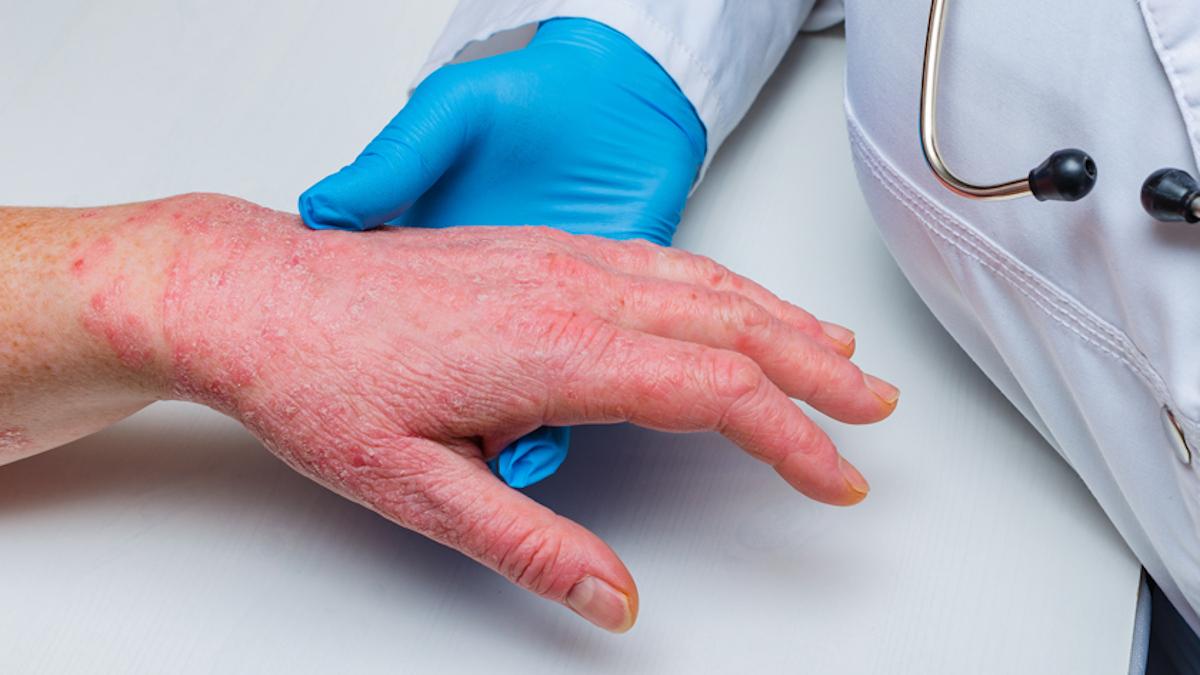
Emboldened by strong data for its oral IL-23 inhibitor icotrokinra in psoriasis, Johnson & Johnson has said it will see if it can outperform its big-selling injectable therapy Stelara in a head-to-head trial.
The decision to start the ICONIC-ASCEND study of icotrokinra versus IL-12 and IL-23 inhibitor Stelara (ustekinumab) comes on the back of the placebo-controlled phase 3 ICONIC-LEAD study, which was presented over the weekend at the American Academy of Dermatology (AAD) annual meeting.
It will be the first-ever head-to-head study seeking to show the superiority of an oral pill therapy over an injectable biologic in moderate-to-severe psoriasis, said J&J, and comes after icotrokinra showed superior efficacy in the skin disorder to Bristol-Myers Squibb's rival therapy Sotyktu (deucravacitinib), a TYK2 inhibitor in the ICONIC-ADVANCE 1 and 2 studies.
In ICONIC-LEAD, top-line results from which were reported last year, 65% of moderate-to-severe psoriasis patients treated achieved clear or almost clear skin (an IGA score of 0 or 1), compared to 8% of the placebo group at 16 weeks, rising to 74% and at 24 weeks.
Similarly, around 50% of the icotrokinra group achieved a PASI 90 response – which equates to a 90% or better improvement in the area and severity of psoriasis skin lesions – at 16 weeks, compared to 4% of the placebo patients. By 24 weeks, the PASI 90 response was 74%, and around half of all patients taking J&J's drug had completely clear skin.
Meanwhile, in the ICONIC-ADVANCE studies, icotrokinra was able to outperform Sotyktu on a series of endpoints designed to show superiority to Sotyktu at 16 and 24 weeks.
J&J's head of immunodermatology Liza O'Dowd said that the "robust results" with its oral IL-23 drug have the potential to "shift expectations" for patients with moderate-to-severe plaque psoriasis.
For patients, it offers the prospect of a new first-line, oral therapy option, rather than having to take periodic injections with injectables like Stelara and J&J's follow-up IL-23 inhibitor Tremfya (guselkumab), which brought in sales of $10.36 billion and $3.67 billion, respectively, last year.
Sotyktu was the first drug to offer that option and has seen reasonably good sales growth since launch, thanks to its oral dosing and efficacy that rivals the injectable treatments. The drug was launched at the end of 2022 and made $246 million last year, although its early momentum has been pegged back by payer access restrictions in the US market, and analysts have said that BMS' forecast of multibillion-dollar sales is now looking unrealistic.
With icotrokinra now showing stronger data, J&J hopes to turn the screw against Sotyktu and other potential oral rivals with ICONIC-ASCEND – assuming, of course, it can show improved efficacy over Stelara.












