FDA clears first OTC Narcan spray for opioid overdose
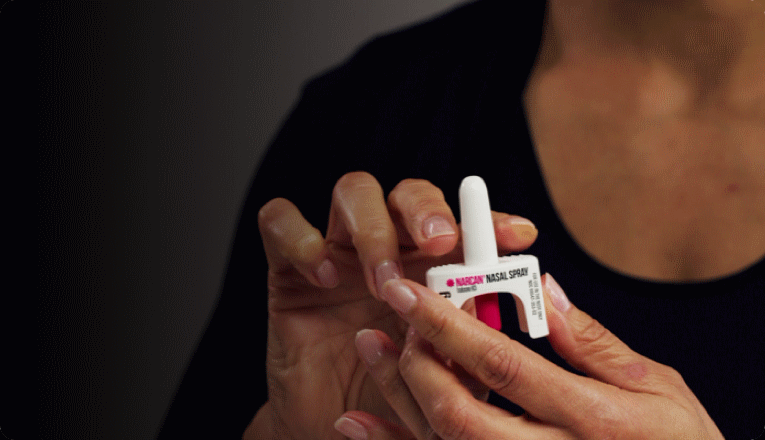
With the US still battling the opioid epidemic, the most widely-used overdose reversal agent – Emergent BioSolutions’ Narcan – can now be bought over the counter at pharmacies, grocery stores, and gas stations, as well as online.
Previously, Narcan (naloxone) 4 mg nasal spray was available through pharmacies and clinics, with almost all US states having laws implementing standing prescriptions, so branded Narcan product or a generic could be dispensed to anyone requesting it without an individualised prescription.
Despite that provision, many pharmacies don’t offer naloxone products, so they don’t have to deal with the social issues that accompany opioid misuse. One recent report examining the consequences of switching naloxone from prescription to OTC use could help expand access, given that retail pharmacies prescribed only around a third of a total of around 17 million naloxone doses in 2021.
The report also showed that bystanders were present, but unable to provide life-saving measures such as naloxone in approximately 46% of fatal overdoses in the US in that year.
FDA commissioner Robert Califf said the switch will increase the number of locations where Narcan is available and help reduce opioid overdose deaths throughout the country.
“We encourage the manufacturer to make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
The opioid epidemic is being driven by an influx of counterfeit copies of established painkillers, like oxycodone, as well as other medicines prone to misuse - like alprazolam, where the active ingredient has been replaced with fentanyl, which is 50 to 100 times more potent than morphine and a major cause of overdoses and deaths in the US and elsewhere.
A recent study in Washington DC found that two thirds of samples of oxycodone tablets seized on the street were found to be counterfeit, and 75% of them contained fentanyl.
More than 100,000 Americans died from drug overdoses in 2021, according to Centers for Disease Control and Prevention (CDC) figures, and fentanyl was considered to be the causative agent in 71,000 of them.
Narcan was first approved by the FDA as a prescription-only medicine in 2015. Emergent said in a statement it would make the nasal spray available on store shelves and at online retailers by late summer, but has not yet indicated how much the OTC product will cost.
One group representing people with substance use disorders, the National Harm Reduction Coalition, called in a tweet for naloxone to be free for everyone in order to ensure broad access and save lives.
The OTC status will also apply to generic versions of naloxone 4mg nasal spray, although manufacturers of those products will have to file for approval to make the switch and update their labelling.












