FDA approves Tandem’s connected insulin controller
Tandem Diabetes Care has claimed approval in the US for a new software system that can be used in “artificial pancreas” systems to automatically check blood glucose levels in diabetics and deliver the appropriate dose of insulin.
The Control-IQ system – which creates a connection between alternate controller-enabled (ACE) insulin pumps and continuous glucose monitoring (CGM) devices – is the first such controller that is flexible enough to be used with a range of devices, and not just those sold by Tandem, says the FDA.
The artificial pancreas – also known as a closed loop control system – was put through its paces in an international trial mainly funded by the US National Institutes of Health that was reported earlier this year.
The study found that using the artificial pancreas system significantly increased the amount of time diabetics spent with their blood sugar in the target range. It is also the first system cleared to deliver automatic correction boluses, in addition to adjusting insulin to help prevent high and low blood sugar.
Tim Stenzel, head of the US regulator’s Office of In Vitro Diagnostics and Radiological Health, said the approval could also allow other controller devices to be reviewed in future via the truncated and recently revamped 510(k) review process, which is used for devices that are “substantially equivalent” to a product already on the market.
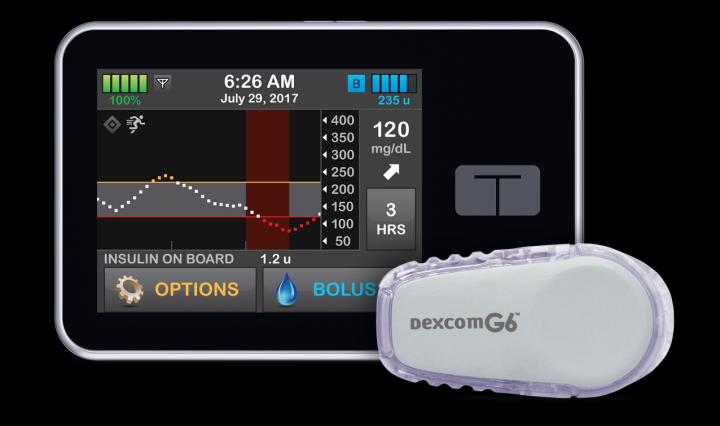
Tandem said the system integrates with Dexcom’s G6 CGM system – which is also being incorporated in personalised insulin delivery systems in development at Eli Lilly – and its own t:slim X2 insulin pump which was approved as an interoperable ACE device in February.
Users of t:slim X2 pumps in the US whose devices are still in warranty will be abler to upgrade to the Control-IQ system free of charge via a remote software update due in January 2020, according to the company.
The FDA approval is for patients with type 1 or juvenile-onset diabetes, an autoimmune form which affects around 5% of all diabetics and must be treated using insulin replacement.
The agency said that it is also establishing “special controls” – regulatory requirements to provide a reasonable assurance of device safety and effectiveness for devices of this type – given the risk of problems such as incorrect insulin delivery as a result of communication problems between devices or hacking.
Control-IQ was derived from a system originally developed at the University of Virginia, by a team led by Boris Kovatchev, director of the UVA Center for Diabetes Technology with funding support from the National Institute of Diabetes and Kidney Diseases (NIDDK).












