EMA panel endorses AI for MASH clinical trials
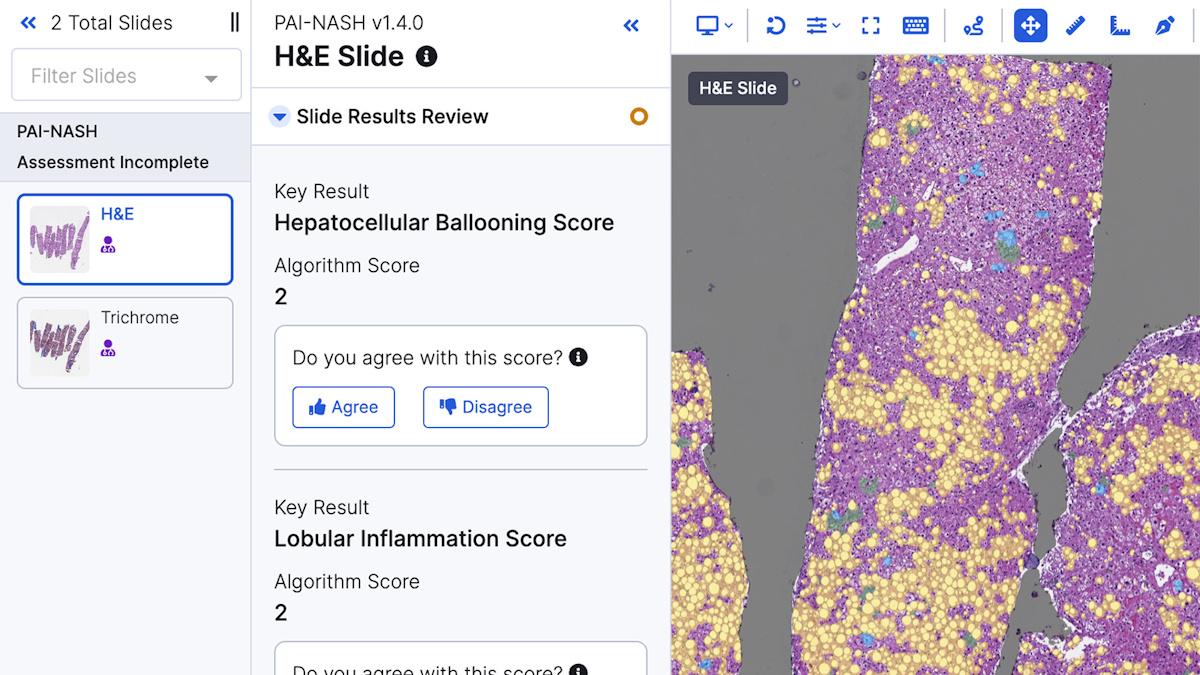
Screenshot of PathAI's AIM-MASH AI Assist in action.
An artificial intelligence model developed by PathAI – used to help diagnose metabolic dysfunction associated steatohepatitis (MASH) from liver biopsy samples – has become the first AI tool to be qualified by the EMA's human medicines committee (CHMP).
The AIM-NASH AI Assist tool "helps pathologists analyse liver biopsy scans to identify the severity of MASH […] in clinical trials," said the EU regulator in a statement.
MASH – also known as non-alcoholic steatohepatitis (NASH) – is a condition where fat builds up in the liver, causing inflammation, irritation, and scarring over time, without significant alcohol use or other reasons for liver injury. It is largely associated with obesity and an unhealthy diet and lifestyle, and is on the rise in industrialised nations.
The CHMP has now issued its first Qualification Opinion (QO) for an AI, agreeing after a public consultation that the AI can increase reproducibility and repeatability in assessments for new MASH treatments, drawing on data from clinical validation study comparing AIM-MASH AI Assist to individual pathologist readings.
That means in turn that it could enhance the reliability and efficiency of clinical trials for new MASH treatments by reducing variability in measuring disease activity and – in theory, at least – lead to treatments becoming available to patients more quickly.
There are no therapies specifically approved for MASH in the EU at the moment, although Madrigal's oral THR β-selective agonist Rezdiffra (resmetirom) – which is already approved in the US – could reach the European market in the latter half of this year. Other drugs, including Novo Nordisk's GLP-1 agonist semaglutide, are also in late-stage development for the disease.
"Testing new MASH treatments often relies on liver biopsies, where small pieces of liver tissue are taken to confirm inflammation and scarring," said the EMA.
"These biopsies are the gold standard for demonstrating the efficacy of new, investigational medicines," it added. "However, high variability in MASH/NASH clinical trials is a challenge, as specialists who review biopsy samples may not always agree on the severity of inflammation or scarring."
AI-powered digital pathology specialist PathAI, based in Boston in the US, said that AIM-NASH AI Assist is designed to assist a single central pathologist in evaluating patients for inclusion in clinical phase 2 and phase 3 trials in MASH, as well as for assessing study outcomes based on histological evaluation.
The company's digital pathology expertise led to an alliance with Roche last year that aims to identify AIs that can be used to match patients with drug treatments and support new drug development.












