ConTIPI starts rollout of pelvic organ prolapse device in US
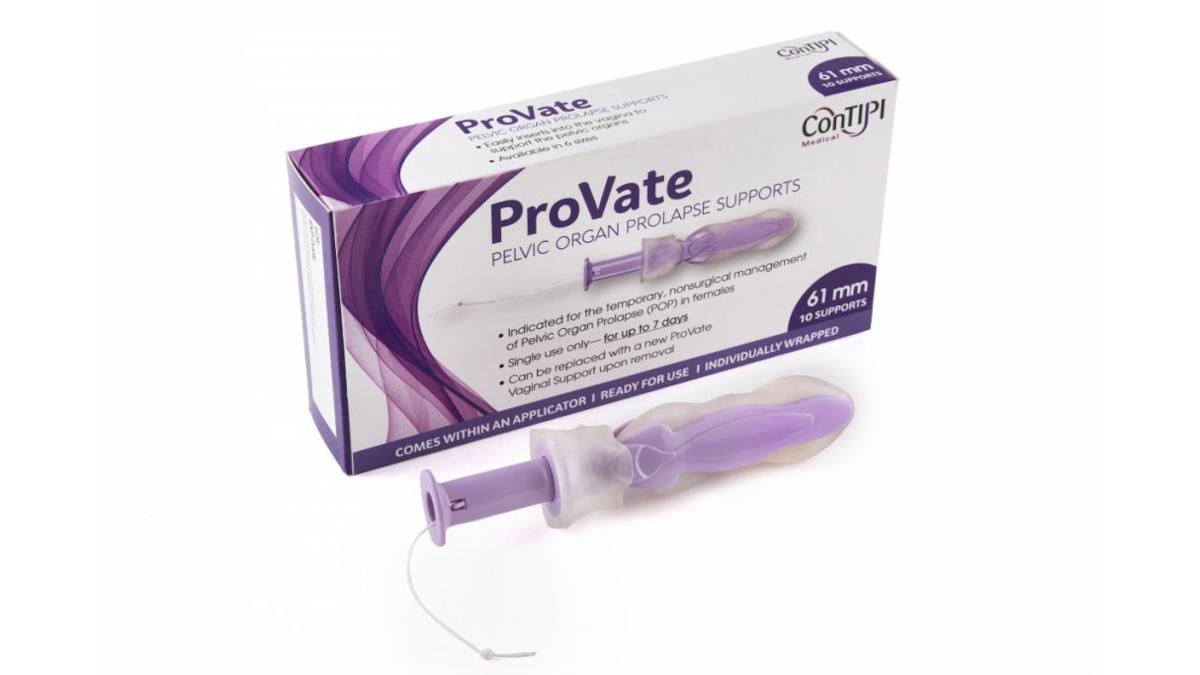
A non-invasive medical device that could help millions of women in the US with a condition known as pelvic organ prolapse (POP) has launched in the US today.
Approximately 57 million American women over the age of 20 years have POP, which occurs when one or more of the organs in the pelvis slip down from their normal position and bulge into the vagina.
In most cases POP causes mild or no symptoms, but it can lead to discomfort and other issues like difficulty urinating and defecating and recurrent bladder infections. It is estimated that around 3.5 million women in the US have symptomatic POP and up to 300,000 undergo surgery for the disorder every year.
The ProVate medical device from ConTIPI Medical is an alternative to current treatment methods, which include invasive surgery – which has a failure rate of up to 30% – or non-invasive but hard-to-use pessary formulations.
According to ConTIPI, existing pessaries have a high discontinuation rate, are difficult for most users to insert and remove, so often require a physician to help, while some women are reluctant to use them because they are reusable devices.
In contrast, ProVate is a single-use alternative that can be used for up to seven days and can easily be replaced with a new support inserted using an applicator - much like a tampon applicator - making it suitable for at-home use. As with tampons, the device can be removed by simply pulling on a string and is available in various sizes.
Once deployed within the vagina, like other ring pessaries, ProVate supports the vaginal walls and mechanically counteracts the prolapse.
ProVate has a 510(k) clearance from the FDA for marketing in the US and also has a CE mark, allowing it to be marketed in Europe.
The commercial rollout in the US is being carried out in partnership with pharmaphorum's parent company Eversana, which will provide commercialisation services including pricing, reimbursement and market access, pharmacovigilance, medical communications, sales, regulatory, marketing, and operational support.
"Today marks a significant milestone in women's health in the US as we are thrilled to see that ProVate is now available for physicians to prescribe for patients with pelvic organ prolapse," remarked Dr Elan Ziv, chief executive and medical director of ConTIPI.
The ProVate Device and the research surrounding it were awarded the Best Novel Therapy presentation during the American Urogynaecology Society (AUGS) meeting in 2017.












