AZ's Zavicefta antibiotic approved in Europe
AstraZeneca's Zavicefta (ceftazidime-avibactam) antiobiotic has been approved by the European Commission.
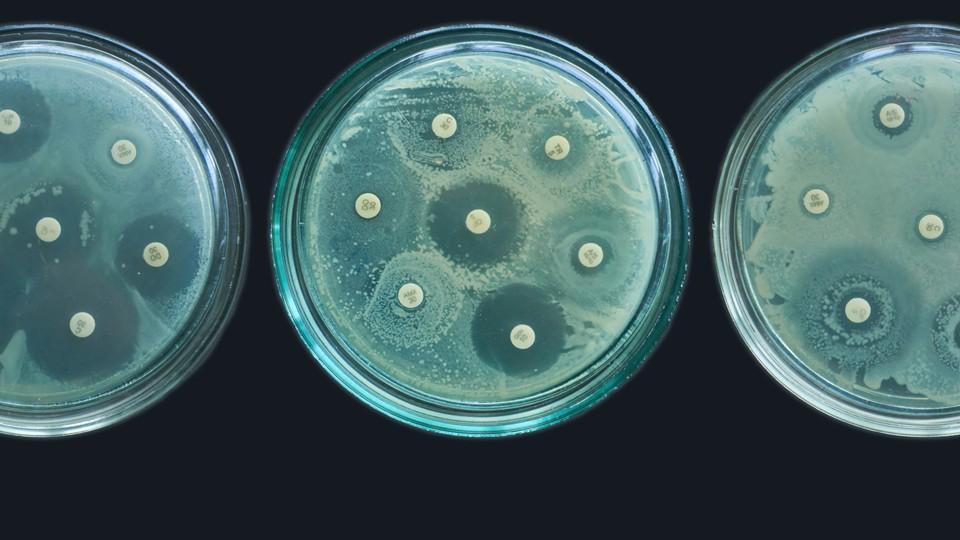
Zavicefta is new combination antibiotic for the treatment of patients with serious Gram-negative bacterial infections requiring hospitalisation.
The approval includes intravenous use of Zavicefta for the treatment of adult patients suffering from complicated intra-abdominal infections; complicated urinary tract infections , including pyelonephritis; hospital-acquired pneumonia, including ventilator associated pneumonia and treatment of aerobic Gram-negative infections in adult patients who have limited treatment options.
Zavicefta has been developed in response to the urgent global need for new antibiotics to treat serious infections that are becoming increasingly resistant, particularly gram negative pathogens.
These include multi-drug resistant P. aeruginosa, carbapenem-resistant Gram-negative pathogens, and ESBL-producing Enterobacteriaceae.
Hans Sijbesma, managing director, AstraZeneca Antibiotics Business Unit, said: “Zavicefta is an important addition to the arsenal of antibiotics in the global fight against antimicrobial resistance. Effective treatment options are rapidly running out for serious Gram-negative infections.
"Zavicefta helps bridge that gap and allows a broad population of patients across Europe to benefit from this new medicine.”
The approval is based on data from an extensive clinical trial programme demonstrating the safety and efficacy of Zavicefta.
The EC marketing authorisation applies to all 28 EU member countries plus Iceland, Norway and Liechtenstein. Zavicefta (ceftazidime-avibactam) is a combination antibiotic that has been developed to treat serious Gram-negative bacterial infections.
It consists of a combination of avibactam and ceftazidime – a third generation antipseudomonal cephalosporin with a well-established efficacy and safety profile. Avibactam is a first-in-class broad-spectrum β-lactamase inhibitor, which protects ceftazidime against degradation by Class A, C and some D, β-lactamases.
A major strategic report on how the world can fight antimicrobial resistance (AMR) was launched last month by Lord O’Neill. The Independent Review on AMR forecast catastrophic consequences if no action was taken: 10 million deaths a year by 2050, a cumulative hit to the world economy of $100trillion, and an undermining of all modern medicine as we know it.
AstraZeneca's approval shows the pharma industry is already responding to new incentives to develop novel antibiotics, but O'Neill recommended a multi-billion dollar fund to help accelerate research and development of drugs and diagnostics.