3D-printed tumours could aid cancer therapy R&D
A 3D bioprinter creates small, three-dimensional tumours in a collagen gel that continue to grow. The researchers then add T-cells and observe what happens.
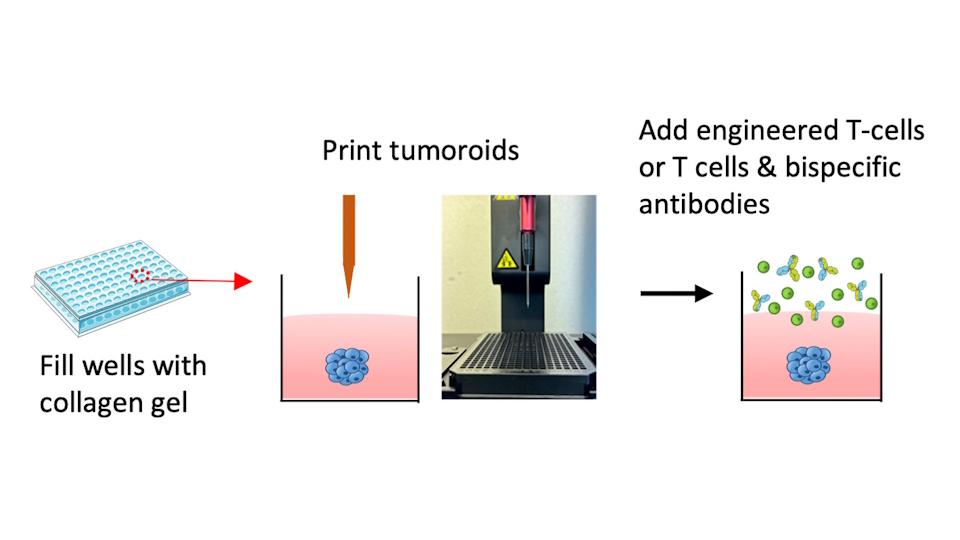
Researchers in the Netherlands have developed 3D-printed 'mini tumours' that behave like real cancerous tissue and can be used to test new treatments, including drug and cell-based immunotherapies.
The team from the Leiden Academic Centre for Drug Research say the models are an alternative to the current approach of testing immunotherapies by culturing tumour cells in a petri dish alongside T-cells, or drugs such as antibodies, and seeing how they interact.
"In a petri dish, T-cells grow among tumour cells and can immediately start killing them," explained Erik Danen, professor of cancer drug target discovery at Leiden. "In reality, T-cells must navigate to the tumour first, which adds complexity."
Their approach involves injecting and embedding "tumouroids" in a collagen gel, which mimics the environment that the tumours would experience in the body more closely and allows the cancer cells to organise into 3D structures that grow and invade into the gel and closely resemble real tumours in the body. T-cells are then added and have to find their way to the tumour.
The 3D printing method is high-throughput and suitable for testing enhanced T-cells and bispecific antibodies, according to the researchers. Meanwhile, the team has also developed a method to monitor real-time interactions of these mini-tumours with immune cells during tests, using automated microscopes, which makes it possible to observe what happens inside and around the tumour and track the immune cells.
That has revealed new insights into the activity of antibodies that were not possible with the conventional approach. For example, during testing with bispecific antibodies, the researchers found that the most effective antibodies not only activate T-cells, but also trigger the production of signalling molecules that attract additional T-cells.
"We can see not only if and how enhanced T-cells and antibodies work, but also investigate the defensive strategies employed by tumour cells," said Danen. "With the old method, the antibodies did not have a chance to reveal this behaviour, because T-cells were mixed with tumour cells and could begin killing them immediately. Our new method will help identify the most effective antibodies for further clinical development."
Anything that could help improve the development of new cancer therapies would be most welcome as, despite significant advances in recent years, the overall success rate of clinical trials remains very low, with the chances of bringing a new therapy to regulatory approval as low as 3% to 5%, according to a recent review paper.
The mini tumours are already being put to work testing Cd3xHER2 bispecific antibodies and evaluating novel T-cell receptor (TCR) therapies that are ready to start human testing for breast cancer.











