Who delays NICE technology appraisals anyway?

Leela Barham looks at the speediness of NICE’s technology appraisals and ongoing efforts to hit the target of NICE publishing final guidance within 90 days of marketing authorisation.
Faster NICE guidance…
Whilst it’s varied over time, NICE has been getting faster at publishing final Single Technology Appraisal (STA) guidance (see figure 1). The most recent public data available from the Department of Health and Social Care (DHSC), NICE’s sponsor department, shows that it took on average 8.25 months from marketing authorisation (MA) for cancer STAs to be published in Q1 2023. It was less than that – 5.71 months – for non-cancer STAs in the same quarter.
Figure 1: Speed of STAs, Q1 2018 to Q3 2023
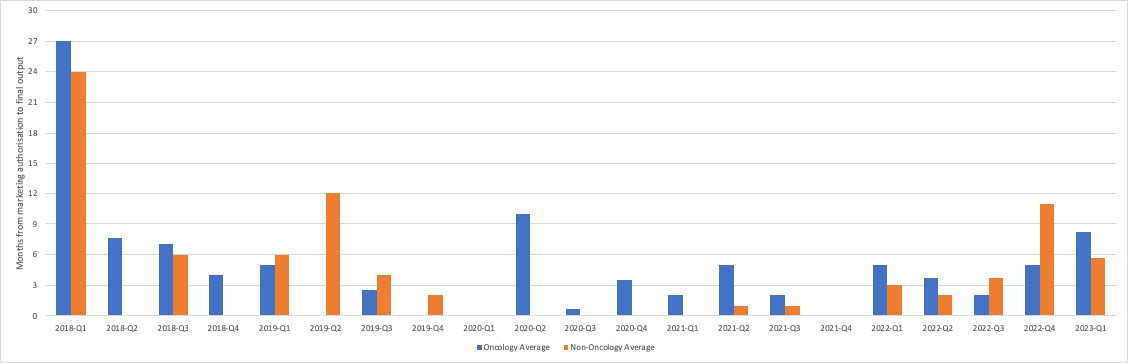
Source: Data supplied by the DHSC in response to a freedom of information request. Data underpins chart in DHSC (September 2023) 2019 Voluntary Scheme Metrics Tracker. Single Technology Appraisals only. Does not include updated TA Guidance. Prior to 2022/23 only includes NAS. From 2022/23 also includes License extensions. Only includes STAs subject to NICE KPI (e.g., referral received on time, company did not request a delay) – in 2019 this was 50% of NAS STAs, in 2020 it was 32% of NAS STAs, in 2021 it was 18% of NAS STAs, in Q1 2022 it was 20% of NAS STAs. In Q2-4 2022, it was 30% of total STAs, and in Q1 2023 it was 37% of total STAs. Where speed is “0” months, difference between MA and first output is less than one month. Quarter refers to final publication quarter. Data to Q1 2023.
Yet, this is only half the story, at best. The data only covers STAs in scope for the NICE key performance indicator. STAs are included where the referral was received on time and the company didn’t request a delay. The proportion of non-KPI STAs - and so aren’t included in the chart - has been rapidly increasing over time. In 2019, the KPI only covered half of NICE STAs, the highest it’s been since 2014. It dropped to just 30% in Q2 to Q4 of 2022 and only reached 37% in Q3 2023.
That’s the first hint that there are definitely factors outside of NICE’s control that slow things down and, it seems, for a lot of their guidance too.
…but still falling short of the target
Leaving aside the rights and wrongs of having a target at all, and whether it’s the right target, NICE aims to publish final technology appraisal or highly specialised technologies guidance within 90 days of the date of marketing authorisation (MA). Based on the DHSC data, NICE has in the past often struggled to achieve the goal.
There’s pressure on NICE to do better. Just on the 8th May 2024, Baroness Delyth Morgan, chief executive of Breast Cancer Now, told an ongoing inquiry by the health and select committee, made up of a group of members of parliament, that “the whole process [at NICE] needs to be speeded up. Because we’ve seen an enormous gap between licensing and drugs becoming accessible.”
Quick win
NICE has already secured a quick win when it comes to speed by introducing a proportionate “light-touch” approach for what the institute describes as “low-risk” treatments.
A first phase of the proportionate approach was introduced in 2022 and six treatments went through it, speeding up those technology appraisals by up to 20 weeks.
A second phase is ongoing, with NICE exploring using the same economic model in appraisals when they are looking at treatments for the same disease. The institute says it is “currently assessing what principles and lessons from the pathway pilot it will incorporate into the STA process.”
Optimal and divergent topics
The institute has also introduced a distinction between treatments; they are now classified as ‘optimal’ or ‘divergent’. Where it’s optimal, the institute can publish guidance within the 90-day window of the GB MA, but not when it’s divergent. Divergent can really be taken to mean “not our fault”.
Optimal is where:
- NICE gets notification more than 16 months before GB MA
- The company accepts the NICE topic selection or routing decision within the agency; this might be whether it’s within the Single Technology Appraisal (STA) route or the Highly Specialised Technology (HST) route
- The company doesn’t negotiate a delayed date for submitting evidence
- Technical engagement is not needed
- Data isn’t submitted past the date of evidence submission
- The incremental cost effectiveness ratio is agreed at the first committee meeting, meaning no consultation is required
- Commercial discussions don’t delay or pause the NICE process, be that before or after the committee meeting
- There’s no appeal for the topic or, if there is one, the appeal points are upheld
- No other external factors can cause delay to the NICE timelines
NICE has far more medicines classed as divergent than optimal: in 2022/23, seven were optimal versus 47 divergent in the same year, according to the institute’s March 2024 Integrated Performance Report.
NICE was also aiming to reduce the mean and median number of days between GB MA and publication of technology appraisals by 15% for optimal topics in 2023/24. It had already achieved a 17% reduction in 2022/23.
Drivers of delays
That NICE has already done work in-house and delivered faster guidance probably explains why the institute is digging more deeply into the external factors that affect the speed at which it can publish technology appraisals. For the first time ever, back in September 2023 the institute published factors that result in less than timely guidance. That analysis was also reported in board papers in December 2023 and again in March 2024.
It turns out that notifying NICE less than 24 months ahead of the GB marketing authorisation was the most frequently identified primary factor impacting NICE’s ability to publish timely guidance (figure 2). The reporting for 24 months - not 16 months to align with the criterion for divergent topics - is likely a hangover from when NICE used to require 24 months’ notice.
Figure 2: Divergent topics: Primary factors impacting NICE’s ability to publish timely guidance, % of all factors
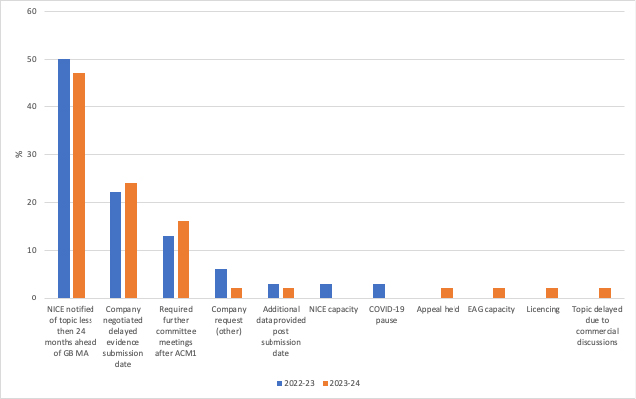
Source: Data from NICE (March 2024) Integrated Performance Report. Available from: https://www.nice.org.uk/get-involved/meetings-in-public/public-board-meetings/agenda-and-papers-march-2024
Delaying evidence submission is the next factor that drives delays. Perhaps surprising is that delays due to commercial discussions is rarely a primary factor.
NICE also noted in the March 2024 Integrated Performance Report that a key risk and challenge to production of timely guidance is the impact of accelerated regulatory processes at the UK regulator MHRA.
HSTs were also singled out for comment. The HST route is intended to be exceptional, applying when the treatment is for a small number of patients, limited or no treatment options, and where there are challenges for research and difficulties collecting evidence, reflecting the uniqueness of the disease. NICE stated that “HST publications (6) have a mean of 1,625 days [from GB MA to final TA/HST guidance publication during 1st April to 31st January 2023], which is significantly affecting the divergent and overall data for 23/24.” That contrasted to a mean of 45 days for 45 TA publications over the same period.
The NICE December 2023 Integrated Performance Report also highlighted a particular HST. HST27 was an appraisal of Clinuvel’s Scenesse (afamelanotide) for treating erythropoietic protoporphyria (EPP), a rare metabolic disorder characterised by phototoxicity. Final guidance was published by NICE in July 2023, not recommending use, but the appraisal had initially been scoped by NICE back in 2016. Along the way to the final guidance were two appeals and three consultations.
NICE is doing more work this year to dig more deeply as to why there are deviations from NICE’s usual timelines, according to Helen Knight, Director of Medicines Evaluation at NICE, who also gave evidence to health and social care select committee.
Ongoing work at NICE
In recognition of the biggest driver of delaying being the need for NICE to know what’s coming sooner, NICE’s chief executive, Dr Sam Roberts, told MPs how NICE “are doing lots of work with industry and the regulator and overseas regulators to rectify that [NICE didn’t know that the treatment was coming in time].”
According to Knight, NICE is also cognisant of ongoing work at NHS England, the buyer of specialist medicines, to revisit a commercial framework and there’s interest in exploring “can […] commercial negotiations happen at an earlier point?” NICE is also working with their independent academic groups, the EAGS, to check that they are “giving [NICE] exactly what we need, at a time that we need it.” Appeals are being looked at too.
Over to others
NICE meeting the 90-day target for publishing final technology appraisal guidance is proving elusive, although the institute is working hard in-house to deliver it. But once the institute has done all it can, the question is what can - and will - others do to help NICE deliver timely guidance?












