Integrating market access considerations into biopharmaceutical asset investment decisions: Common pain points and best practices

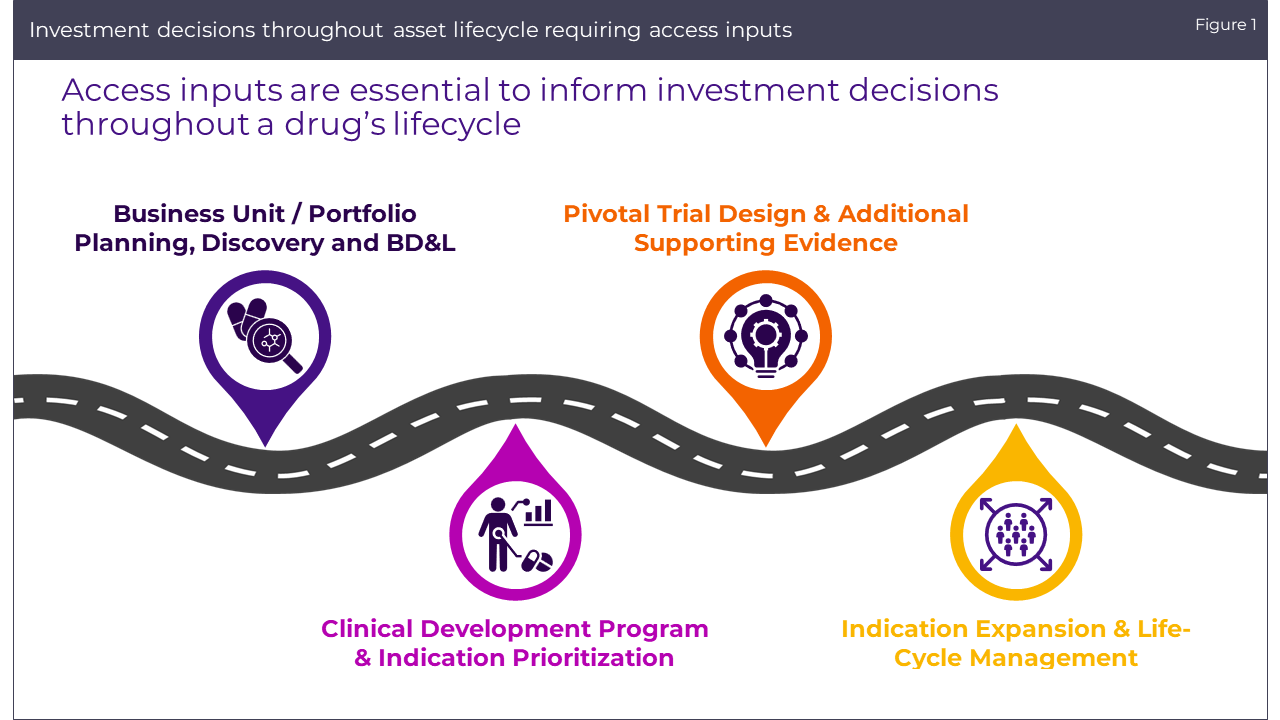
The biopharmaceutical industry operates at the intersection of health innovation and market viability, where the significance of integrating market access insights into investment decisions cannot be overstated. From the inception of a new therapy to clinical development and pivotal trial designs, market access analyses provide a critical lens through which the commercial trajectory of a product is evaluated. This approach ensures not only the development of clinically effective products but also their market success by aligning them with payer expectations and healthcare systems’ needs. As the industry faces growing complexities, the ability to navigate these with informed access integration becomes imperative for reaching patients effectively.
In our work with clients, we have experienced several common organisational pain points in integrating market access into investment decision-making, and we have identified and replicated best practices for mitigating these challenges.
Common pain points in integrating access voices into investment decisions
The path to integrating market access considerations into pharmaceutical asset investment decisions is fraught with challenges that can hinder effective decision-making:
Inefficient and unclear objectives:
Market access teams may find themselves navigating without a clear direction, working towards objectives that are not only vague but also inconsistently applied across different stages of the process. The focus often shifts between assessing static Target Product Profiles (TPPs) and trying to develop a singular “reimbursable” TPP, without considering the dynamic nature of market access environments and the multitude of potential pathways a drug development process could take.
• Read the full article in pharmaphorum's Deep Dive digital magazine












