Oncology trials: Top 5 trends to watch
From innovative trial designs to groundbreaking imaging techniques, oncology studies are rapidly evolving. Advanced Clinical’s VP of oncology, Ravi Karra MD, MBA, explores the top trends shaping the future of oncology clinical trials.
The oncology clinical trial landscape is undergoing a profound transformation, driven by the rapid pace of pharmaceutical and biopharmaceutical innovation in cancer research. As new and advanced therapy options continue to emerge, such as cell and gene therapies and immunotherapies, the enthusiasm surrounding oncology clinical trials and the promise they hold for patients is reaching new heights.
The emergence of innovative new drug classes, along with improved imaging techniques, is reshaping the way clinical trials are designed, conducted, and analysed. This ultimately contributes to steady improvements in overall survival (OS) and progression-free survival (PFS) for patients across a wide range of cancer types.
Here, we delve into the key trends shaping oncology clinical trials and examine the innovative trial designs and cutting-edge techniques that are set to optimise patient outcomes and accelerate the path to new cancer treatments.
Trend 1: Trial designs are changing
The traditional linear and sequential approaches to clinical trials are giving way to innovative, adaptive, and flexible methodologies. These new trial designs are crucial in addressing the complex demands of modern oncology treatments, aiming to accelerate the development of life-saving therapies. Adaptive trial designs allow researchers to dynamically modify trial parameters based on interim results, utilising real-time data. This flexibility minimises the number of required participants and shortens trial durations, making the process more efficient and effective. If early efficacy data shows a higher-than-anticipated response rate, researchers can adjust the sample size accordingly, reducing unnecessary exposure to ineffective treatments and expediting the development timeline.
Basket trials evaluate a drug's effectiveness against a specific mutation across multiple cancer types. This approach enhances the applicability of findings, enabling a single treatment to be explored in various contexts. For example, a drug targeting the BRAF mutation can be concurrently tested in patients with melanoma, colorectal cancer, and thyroid cancer – consolidating research efforts and potentially accelerating the availability of effective treatments.
Just as basket trials explore a drug's potential across different cancers, umbrella trials take a complementary approach. Umbrella trials involve testing multiple drugs on various mutations within a single cancer type. This method streamlines the process for complex cases by combining multiple treatment pathways under one protocol. In colorectal cancer, for instance, diverse treatments targeting various genetic mutations can be evaluated simultaneously, fostering a comprehensive understanding of the disease and its potential therapies.
Trend 2: Transforming early-stage clinical development
Complementing the paradigm shift in clinical trial designs, early-stage clinical development is evolving to incorporate more sophisticated and targeted approaches, focusing on rapidly identifying promising drugs based on patient-specific factors and biological markers.
Traditionally, Phase 1 oncology trials solely evaluated maximum tolerated dosages of harsh chemotherapies. But that model is evolving for today's targeted therapies and immunotherapies. What used to be Phase 1 – purely a safety trial assessing maximum doses – has moved on. The new focus is on determining the optimal biological dose: the level that can effectively attack a patient's tumour while remaining tolerable.
Rather than just toxicity, these new biomarker-driven early trials aim to identify the ideal therapeutic dose matched to each patient's specific biology from the start. Novel designs like the Bayesian Optimal Interval approach are replacing the former "3+3" standard, allowing smarter and faster dose evaluations, aligned with precision medicine.
The shift in early trial methodologies reflects the broader transformation occurring in oncology drug development. Biologics have gained significant traction, encompassing innovative cell and gene therapies where genes are manipulated ex vivo or in vivo. Treatments like CAR-T cell therapies targeting a patient's own re-engineered immune cells and drugs inhibiting specific mutations are now routinely explored from the earliest clinical stages.
By evolving Phase 1 methodologies to focus on optimal biological dosing and patient biomarkers upfront, we can more efficiently prioritise modern oncology's most promising therapeutic candidates. More innovative early trial designs match the increased scientific complexity, aligning these crucial initial human studies with a personalised future for cancer therapy. Reforming our early development paradigms will be essential to translating advances in biology into patient impacts.
Trend 3: The rise of new drug classes
While chemotherapies were once our primary arsenal, oncology has radically expanded its therapeutic horizons over the past couple of decades. We have transitioned from simply relying on small molecule chemicals to harnessing the power of large, complex biological molecules and even re-engineering living cells themselves.
What once seemed like science fiction is increasingly becoming standard-of-care at major cancer centres across Western Europe and North America, particularly for haematological malignancies. Novel therapeutic classes, such as immunotherapies, are leading this transformation.
This shift is reflected in regulatory designations, such as the European Medicines Agency's "Advanced Therapy Medicinal Products" (ATMPs) category, which encompasses groundbreaking modalities, including cell and gene therapies involving ex vivo or in vivo manipulation of patient genes or cells. Under this approach, a patient's immune cells can be extracted, bioengineered to selectively recognise and attack their specific cancer, and then reinfused back into the body as a living therapeutic to eradicate tumours from within.
Notably, immune checkpoint inhibitors function as a sort of "brake release" - allowing the body's own T-cells and other defences to vigorously attack cancer after the tumour's immune-evasion mechanisms are blocked. Since malignancies originate from the patient's own cells, they employ sophisticated camouflage tactics. Checkpoint inhibitors strip away that disguise so that the cancer can no longer hide.
Alongside immunotherapies, we have seen a boom in precision-targeted therapies designed to hit specific genetic drivers, like BRCA mutations, in breast cancer. When a patient's tumour harbours that molecular aberration, these rational drugs can provide exquisitely targeted and effective treatment by directly inhibiting the root cause.
The oncology pipeline has rapidly expanded beyond our former chemotherapy horizons, from re-engineered living cells serving as therapeutics to genomically targeted magic bullets and immunomodulators. Continued research, development, and innovation across these diverse modalities will be vital for sustaining transformative advances against cancer.
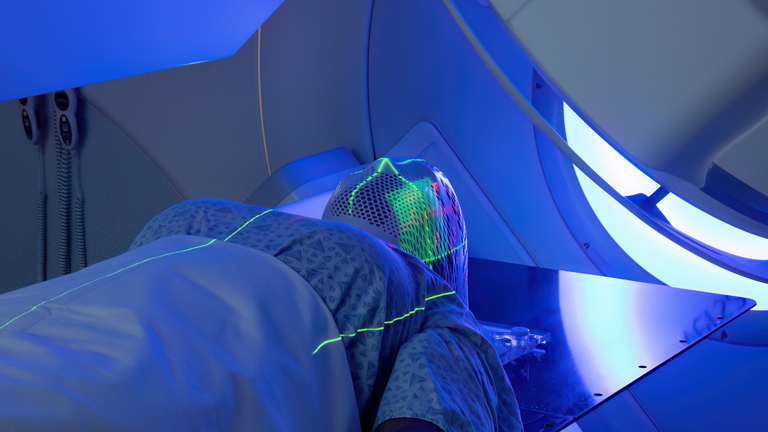
Trend 4: Innovation in imaging techniques
While techniques like X-rays, ultrasounds, CT scans, and MRIs remain vital for detecting tumour masses and monitoring changes over time, these anatomical imaging modalities provide limited insight into the underlying tumour biology and response to treatment. The focus has been on visualising physical characteristics like mass dimensions and location.
This landscape is evolving with the emergence of molecular and functional imaging techniques that can visualise tumours at the molecular and metabolic levels. For example, positron emission tomography (PET) scans using radiolabelled glucose tracers like 18F-FDG can highlight the distribution of viable, metabolically active malignant cells based on their elevated glucose uptake compared to surrounding tissue.
Molecular imaging also enables more precise cancer phenotyping and targeted treatment selection. For prostate cancer specifically, PET tracers targeting prostate-specific membrane antigen (PSMA) overexpressed on malignant cells enable visualisation of disease based on this biomarker phenotype. Rather than simple shape estimates, these scans map the precise distribution and burden of viable PSMA-expressing lesions throughout the body with high resolution.
Beyond visualising metabolic activity, molecular imaging techniques can expose other critical cancer hallmarks, such as angiogenesis, hypoxia, proliferation, and treatment resistance mechanisms. Information that was previously inaccessible is now discernible through these advanced, mechanism-based visualisation approaches.
As molecular profiling and biomarker-guided therapies become increasingly integral to personalised cancer care, these advanced imaging platforms complement anatomical techniques by providing additional context about each patient's specific tumour biology and treatment response patterns. Integrating molecular and anatomical visualisation methods enhances diagnostic, staging, and treatment monitoring capabilities across oncology.
Trend 5: Optimising existing cancer treatments to suit patient profiles
Historically, the relatively small patient populations impacted by rare cancer types posed challenges for driving substantial research and drug development investments from biopharmaceutical companies. With high costs and extended timelines for oncology product development, companies generally needed to project broad potential markets to ensure financial viability following approval.
However, that landscape is shifting, due to new regulatory policies specifically aimed at incentivising therapies for rare diseases. Agencies like the FDA and EMA have implemented programmes granting extended market exclusivity periods of eight to ten years to sponsors developing rare disease treatments. These protections provide sufficient time to establish new medicines and recoup investments before biosimilar competition emerges.
As a result, clinical trials are increasingly open to explore both novel agents and existing approved drugs that may have utility for new rare cancer indications. Strategies like repurposing or repositioning can help rapidly expand treatment options for underserved populations by circumventing portions of the standard drug development pathway.
Optimising available treatments by choosing the best sequence of first, second, and further lines of therapy, alone or in combination, based on patient-specific factors, represents a truly personalised approach. Ongoing efforts to refine and enhance treatments for widespread cancers, like colorectal and breast cancer, are critical and illustrated by new combination treatments and better sequencing of treatment lines, which offer improved outcomes with fewer side effects. For example, integrating novel targeted therapies with traditional chemotherapy regimens can enhance efficacy while minimising toxicity.
Driven by these new financial incentives and scientific approaches, the oncology pipeline is expanding to encompass more therapies for rare tumour types that were historically deprioritised. This policy-driven shift is catalysing a treatment landscape transformation for patients with uncommon cancers.
About Advanced Clinical
Advanced Clinical is a clinical development and strategic resourcing organisation committed to providing a better clinical experience across the drug development journey. Our goal is to improve the lives of all those touched by clinical research – approaching each opportunity with foresight, character, resilience, and innovation. Based on decades of experience, we help our clients achieve better outcomes by conducting candid conversations and anticipating potential issues through our customised solutions.
Visit our website to learn more: www.advancedclinical.com.
About the author
Dr Ravi Karra is a clinical developer in oncology with over 20 years’ experience in senior medical roles in academia and industry including pharma and CROs. He is an expert in brain tumours, melanoma, head and neck cancer, and has been a part of several transformative trials like adjuvant Ipilimumab therapy in Stage III melanoma (Eggermont AMM et al. Lancet Oncol. 2015 May;16(5):522-30) and comparing TPF to PF in patients with unresectable squamous cell carcinoma of the head and neck (Vermorken J. B et al. J. Clin. Oncol.2011 29:15_suppl, 5530-5530). He also led trials in rare and difficult to treat cancers like metastatic uveal melanoma (Leyvraz S et al. Ann Oncol. 2014 Mar; 25(3): 742–746).
He has actively participated in designing and developing innovative trial methods using translational research including molecular and imaging biomarkers, as well as the "Window of Opportunity" concept across tumour types, including rare tumours such as glioblastoma and salivary glands. Dr Karra has extensive experience dealing with the FDA, EMA, and payers for reimbursement, and has been part of successful NDA and ANDA applications and market launches.
Supercharge your pharma insights: Sign up to pharmaphorum's newsletter for daily updates, weekly roundups, and in-depth analysis across all industry sectors.
Want to go deeper?
Continue your journey with these related reads from across pharmaphorum
Click on either of the images below for more articles from this edition of Deep Dive: Oncology 2024