A trusted partner to support clinical research

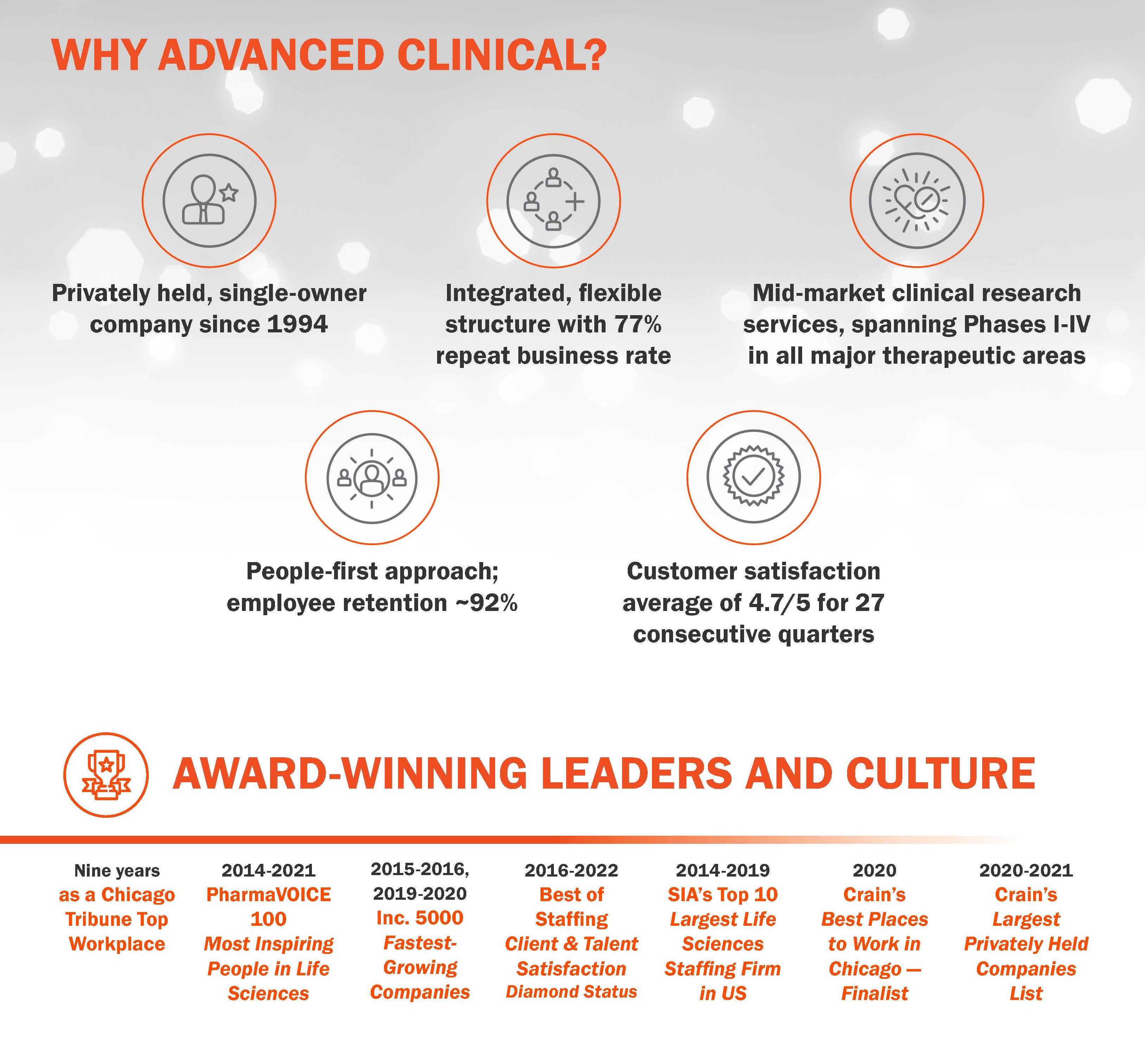
Advanced Clinical is proud to be a privately-held, single-owner company, which was founded in 1994 and has grown organically—not by merger or acquisition—into an award-winning culture, trusted by our clients and internal workforce to deliver a better clinical experience.
Whether planning to launch a clinical trial or seeking the next career step, our organisation is designed to deliver a better clinical experience.
FAST FACTS:
CEO/President: Julie Ross
Office Locations: North America: United States and Canada; Central and Eastern Europe: Poland, Ukraine, Romania; Western Europe: United Kingdom, France, Netherlands, Spain, Ireland, Italy, Germany, Switzerland; Asia-Pacific: Australia, Japan, Singapore
Global Reach: Over 35 countries across the globe including regional partnerships with organisations that support our delivery of clinical research services.
Number of Employees: 500-1,000
Year Established: 1994
Areas of Focus: CRO, FSP and Strategic Resourcing solutions across Phases I-IV
Therapeutic expertise: Over 70% of our therapeutic expertise is in Phase II-III. Experience includes all major therapeutic areas, including drug, biologic, diagnostic, and device with specialised focus in Rare Diseases, Oncology, and Dermatology.
As a global clinical research services organisation providing CRO, FSP and Strategic Resourcing solutions to drive better outcomes across the drug development journey our goal is to improve the lives of all those touched by clinical research—approaching each opportunity with foresight, character, resilience and innovation. Based on decades of experience, we help clients achieve better outcomes by conducting candid conversations and anticipating potential issues through our customised solutions.

Customised CRO solutions for every study challenge
Along with closely evaluating all aspects of clinical trials to proactively assess the best options for client studies, our global team offers a highly collaborative environment and integrated approaches that enable client teams easy access to senior experts and resources from across the organisation, improving the clinical trial experience. Our team works with clients to provide high-quality solutions for all types of clinical research engagements, including the following:
• Site Identification & Selection
• Global Study Startup
• Patient & Site Engagement
• eTMF & Document Management
• Clinical Monitoring
• Medical Writing
• Hybrid & Decentralised Trial Solutions
• Medical Monitoring
• Safety & Pharmacovigilance
• Quality & Validation
• Supply Chain Management
• Therapeutic Expertise (with specialised focus in Oncology, Dermatology, Rare Disease)
Over 25 years of custom-built FSP solutions
As a company expands, knowing how and when to deploy the right resources at the right time becomes critical to driving sustainable growth. It is no secret that there is a talent access problem today across multiple industries, and life science companies are not immune to this problem. There are more roles to fill than qualified people to fill them. More than 70% of life sciences CEOs say they’re worried about key skill shortages, and more than two-thirds report difficulty recruiting talent with the required skills.
Increased research and development, combined with an industry-wide talent shortage, requires a deliberate strategy to address the complex support demands of clinical development programmes.
Advanced Clinical’s functional service provider (FSP) delivery model is powered by our team of experts to create functional solutions that are tailored to the specific requirements of clients' organisational and clinical development strategy. For a true functional service partnership, the combination of our deep understanding of a client's culture and organisational infrastructure with our industry insights and focus on innovation enables us to build solutions customised to their strategic business requirements.
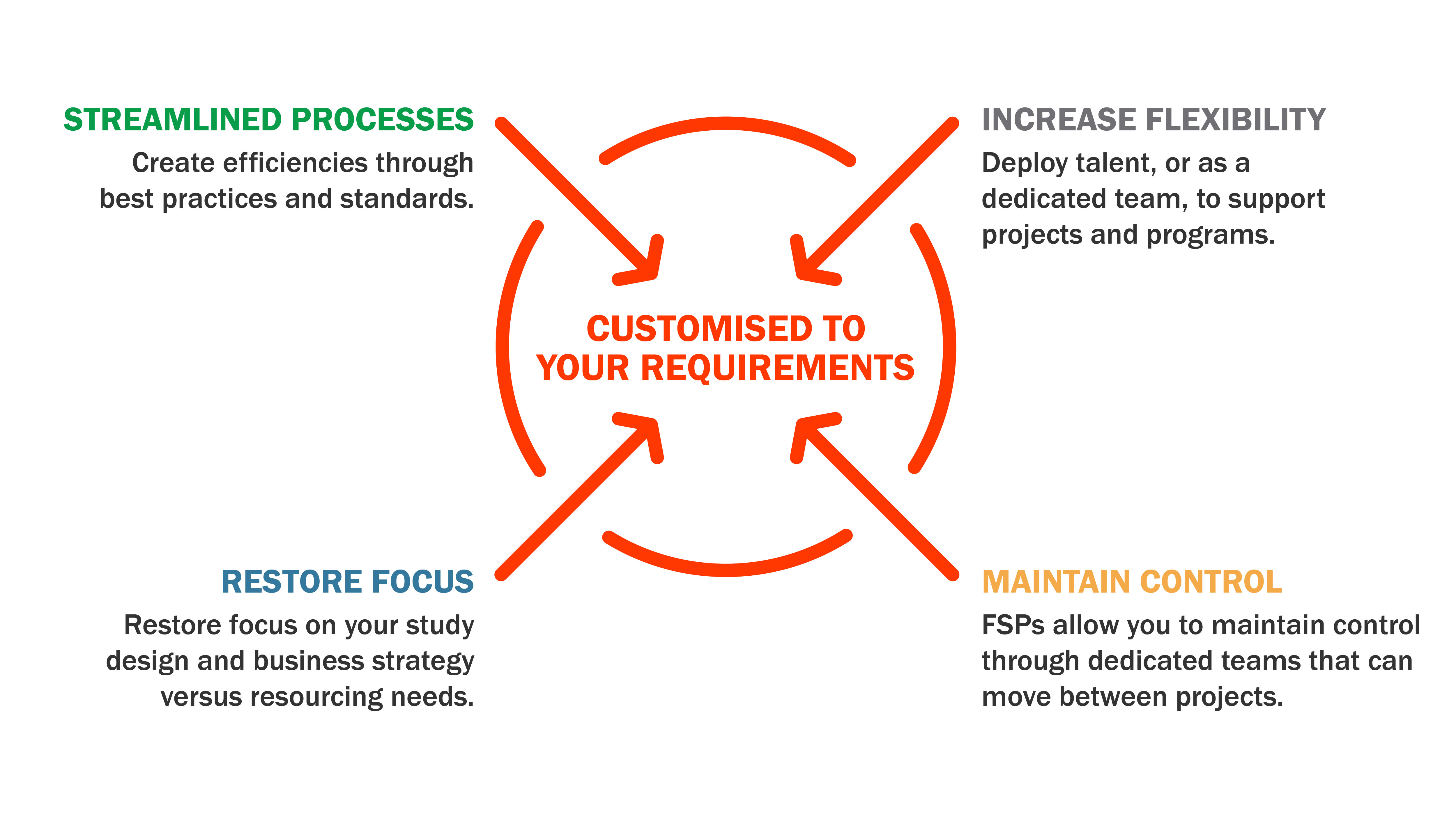
Customised solutions for strategic resourcing
Every day, through each interaction with sponsors large and small, we understand clients want to work with a firm who specialises exclusively in the life sciences industry and partners only with sponsor companies — biotech, pharmaceutical and medical device — with the goal of right-sizing client teams and drawing from our diverse talent network to fill positions with efficiency, with quality in every aspect of clinical research remaining our utmost priority. We work to design and develop resourcing plans to meet their needs, offering such varied and creative solutions as on-demand contingent resourcing, direct hire and permanent placements, contract-to-hire, on-site talent management, workforce planning, and optimisation or payroll support.
• Recruiters average 15+ years of clinical research recruitment experience
• We closely support clinical research talent to help guide career decisions, creating more engaged and loyal candidates
• We’re the preferred resource for candidates, with a repeat placement percentage 8x higher than the industry average
• Screening process and automated engagement tools (SenseHQ® and Vettd.ai®) increase retention and engagement
• Proprietary talent database with 500,000+ candidates
• Clinical Technology Systems (EDC, TMP, Validation)
• Programme Management and Project Leadership
• Software Development
• Cloud Services
• Data Solutions & Business Intelligence
• Infrastructure Support
A focus on diversity to pave the way for more inclusive trials
Having diverse patient populations is crucial to having a successful clinical trial that accurately reflects all people with unmet medical needs. Our team guides clients through clinical trial and decentralised clinical trial (DCTs) experiences with solutions that are customised to their requirements and in a way that is both site- and patient-centric to increase their trials’ probability of success.
Using innovative digital tools to power clinical trials, we understand the benefits DCTs can bring by increasing access to more diverse patient pools, clinical study teams, patients and investigative sites. Our team works with clients in order to identify the following:
- Patient-centric strategies: Proactive plans to address all patient needs— the guiding force for all aspects of trials
- Hybrid and decentralised trials: Reduce patient burden and promote patient-centricity
- Patient support: Adapted technology, travel concierge—and more—to help patients and their loved ones
- Diverse patient populations: Patient engagement and enrolment to diversify patient group for a trial
Partner with us today. Learn more at www.advancedclinical.com











