UK pharma stays ambitious despite Brexit, looks to new era in cell therapy
A momentus week has seen Gilead acquire Kite, while Novartis gained the first CAR-T approval. The UK can be a leader in cell and gene therapy, but Brexit is a once-in-a-generation challenge
While many of us are still rousing ourselves from a long languid summer, this week the pharma sector woke up with a jolt thanks to some hugely significant milestones.
On Monday, Gilead, announced it was acquiring cell therapy and CAR-T specialists Kite Pharma for just shy of $12 billion – a bold, well-timed move which suddenly opens up new avenues for a company which had become a victim of its own success in hepatitis C.
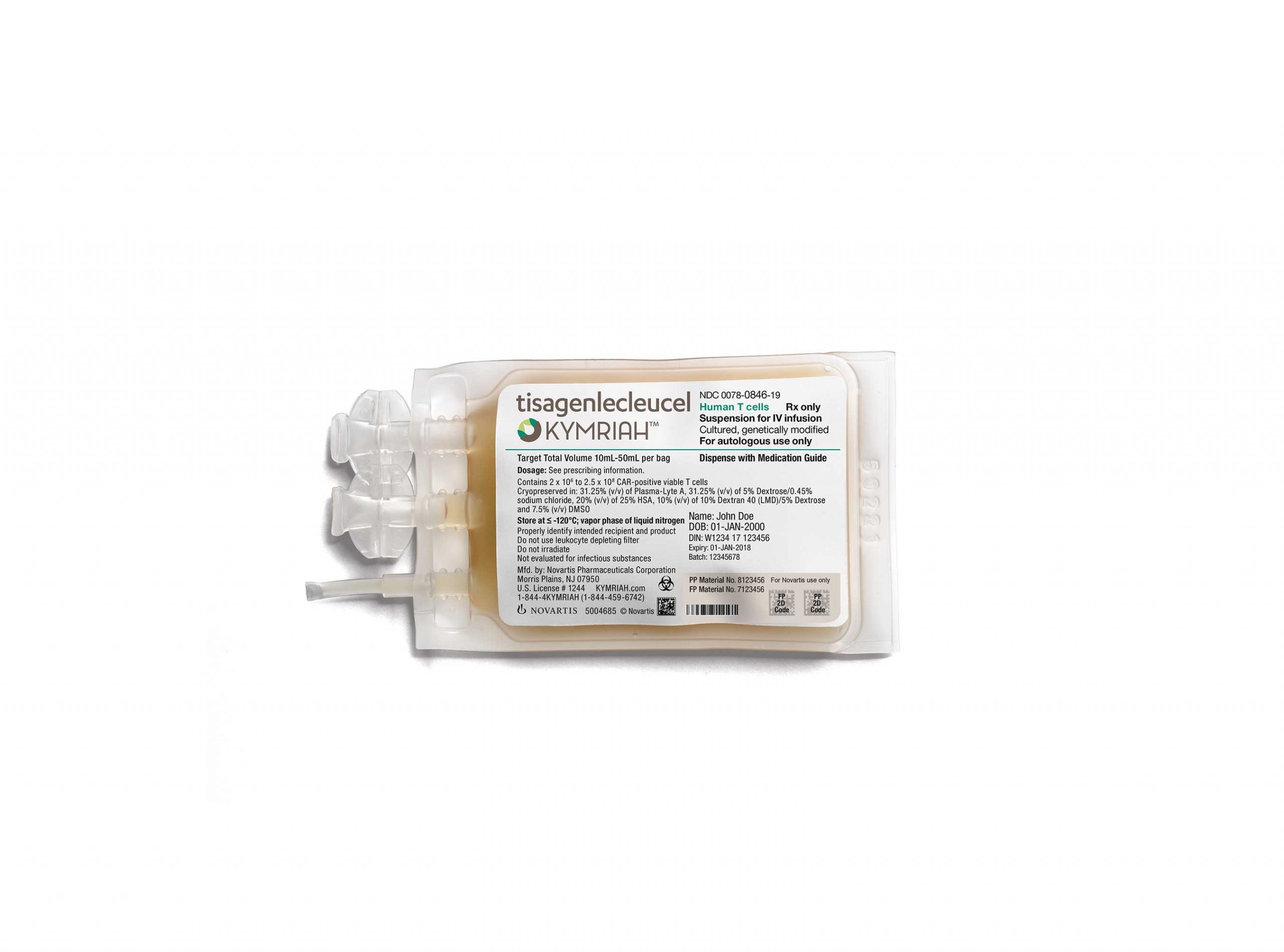
This was followed swiftly yesterday with an earlier-than-expected announcement from the FDA, declaring that Kite’s rival, Novartis, had gained the world’s first approval of a revolutionary CAR-T drug, now named Kymriah.
[caption id="attachment_31240" align="alignnone" width="320"]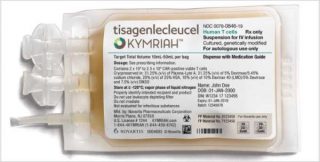 Novartis' groundbreaking Kymriah[/caption]
Novartis' groundbreaking Kymriah[/caption]
And in the UK, yesterday also saw the unveiling of the Life Sciences Industrial Strategy, an industry- led vision of how to supercharge the sector over the next decade, whatever Brexit throws at it.
UK aims to be world leader in cell and gene therapy
On a global (US-led) level, these developments illustrate a biopharma sector flourishing and pushing boundaries thanks to cutting edge science and plentiful cash.
But for the UK, there is also a clear thread linking all of these developments: Kymriah’s approval signals that the era of cell and gene therapy has most definitely arrived, and Britain hopes it can be a world leader in the development and manufacturing of these new highly complex medicines.
Most significantly, the UK sector and government believe becoming a leader in cell and gene therapy could be just the 'good news story' the country needs after Brexit left the rest of the world puzzled, and UK life sciences in limbo.
And one UK biotech company is in the vanguard alongside Novartis: Oxford BioMedica is the sole manufacturer of the lentiviral vector technology used to create Kymriah’s genetically modified T cells.
This exclusive deal could potentially see Oxford BioMedica earn more than $100m from the Novartis collaboration over the next three years, as well as undisclosed royalties on potential future sales of Novartis CAR-T products.
The Kymriah approval is just the tip of the iceberg: the International Society for Cellular Therapy (ISCT) estimates that there are currently more than 40 companies developing redirected T cells or NK cells for therapeutic use, with more than 800 cell therapy clinical trials currently underway.
The UK is already well positioned to be at the forefront of this revolution, which will not only require next generation biotech manufacturing and regulation, but also development of dedicated gene therapy centres and specialist healthcare professionals.
To open up a lead in this field, the UK has numerous initiatives in place: a government funded Cell and Gene Therapy Catapult, and a dedicated Medicines Manufacturing Industry Partnership (MMIP). The MMIP worked closely with the lead author of the newly launched Life Sciences Industrial Strategy, Sir John Bell.
[caption id="attachment_31180" align="alignnone" width="296"] Sir John Bell[/caption]
Sir John Bell[/caption]
This means that cell and gene therapy manufacturing is at the heart of the proposed industrial strategy. While the government is hanging back from committing money to other fields, yesterday it confirmed that £146 million would be spent across five major projects supporting advanced therapies, advanced medicines and vaccines development and manufacturing.
The Brexit clock is ticking
But next generation manufacturing is only one part of the complex, interconnected life sciences ecosystem.
The Life Sciences Industrial Strategy addresses this in a well-defined, intelligently assembled set of long-term goals. These could certainly boost the UK’s £64 billion life sciences sector if fully implemented.
However its most ambitious goals are likely to be hostage (at least in part) to Brexit, and the regulatory disarray and economic downturn it could trigger.
The risks and complexities of exiting the EU by April 2019 are so huge that the report hasn’t tried to tackle them head on in the main.
The report does state however that: “If managed carefully, EU exit may be used as a catalyst to take steps to speed the growth of the life sciences sector in the UK.” This is a carefully worded phrase, and is a clear challenge to government to follow up on its promises of support.
However it also skirts the real possibility that Brexit will set the sector back considerably – from leaving the EMA regulatory network, to losing EU science funding and deterring scientific and business talent coming to the UK.
This is also against a background of limited funds for government investment in R&D funding and in the NHS. Funding for new medicines is the big sticking point between industry and government today, and the new plan hasn’t found any new ‘easy wins’ on this vexed question.
Nevertheless, relatively modest funding can make a big difference to R&D infrastructure and NHS innovation projects if they are well targeted and managed- the report identifies ongoing investment in genomics and the potential of big data.
However on the question of pharma regulation, the report – which draws mainly on industry expertise – makes it clear that bold ideas about the UK ploughing its own furrow in pharma regulation won’t pay off.
It notes: “There has been much discussion about the opportunity of the UK to develop innovative regulatory approach to emerging technologies outside of the EU,” but says while this might be ‘theoretically desirable’ the loss of EMA alignment would be a far greater loss than this gain.
It adds: “Relatively speaking, the UK market is too small even with the fastest and most innovative regulatory system in the world, to stand alone from a larger decision-making bloc.”
This sober assessment of the UK’s position will have to be retained alongside the laudable ambitions laid out in the strategy, so that the UK life sciences sector can retain its momentum regardless of the many potential pitfalls of Brexit.
Read the full Life Sciences Industrial Strategy here












