Site quality management: the revolution in risk-based monitoring

Joshua Pines and Stephen Young
Medidata Solutions Worldwide
Joshua Pines and Stephen Young offer advice on how should your organization proceed with implementing a risk-based site monitoring strategy.
It is by now well documented that the industry is actively exploring a paradigm shift in its approach to ensuring quality in the conduct of clinical trials. In particular, a more targeted, risk-based approach to site monitoring and data cleaning is being pursued or explored by numerous clinical R&,D organizations. The primary goals of this paradigm shift are to significantly improve the resource efficiency associated with clinical R&,D, increase the quality achieved in trial conduct and corresponding data, and maintain or reduce clinical trial timelines.
The time is now
A number of clinical R&,D solution providers have already started delivering software and services to enable and support the successful implementation of risk-based site monitoring strategies. Given the recent FDA guidance firmly endorsing these strategies, it is becoming apparent that now is the time for both sponsors and their contract research organization (CRO) partners to fully embrace the new paradigm.
So how should your organization proceed with implementing a risk-based site monitoring strategy? An effective strategy may take on a number of different forms but should always include the following components:
• A reduction in source document verification (SDV) coverage that is targeted (or weighted) toward the most critical data and higher risk sites.
• A method of remotely and centrally monitoring site and data quality in a manner that can proactively alert study teams to potential emerging quality issues at one or more sites.
• Less frequent on-site monitoring visits, enabled by the remote monitoring capability and significantly lower SDV burden.
• Periodic “phone visits” to sites between on-site visits.
• Study monitoring plans that describe all methods needed to assess site quality, including remediation / corrective actions to be taken when issues are observed.
 ,
"...the industry is actively exploring a paradigm shift in its approach to ensuring quality in the conduct of clinical trials."
 ,
From a technology solutions perspective, use of electronic data capture (EDC) should be considered a prerequisite for this strategy. EDC is not necessarily the only system providing operational data descriptive of site quality, but it is inevitably the most robust source of such information available remotely and proactively during study conduct. Since EDC is a key enabler, it is very important that EDC solutions be effectively equipped to manage the planning and execution of a partial SDV plan, especially as EDC is the tool within which SDV is managed and tracked. Optimally, such a solution will enable your study teams to dynamically change the SDV requirements for a given site during the trial, based on quality findings at that site. This may include increasing — or even decreasing — the level of required SDV on a site-by-site basis, allowing teams to target their limited resources to the sites needing the most attention, which in turn leads to increased quality across the study.
Optimizing site quality is key
A key additional capability required by sponsors to more effectively manage a risk-based monitoring strategy is the ability to remotely and centrally view and assess various site and data quality measures in a way that quickly and effectively draws attention to sites with emerging quality issues. We can refer to this capability as site quality management (SQM). An SQM solution should proactively identify sites with potential emerging issues across a number of relevant site quality measures for an ongoing clinical trial. Figure 1 lists examples of site quality measures that organizations should be interested in tracking:
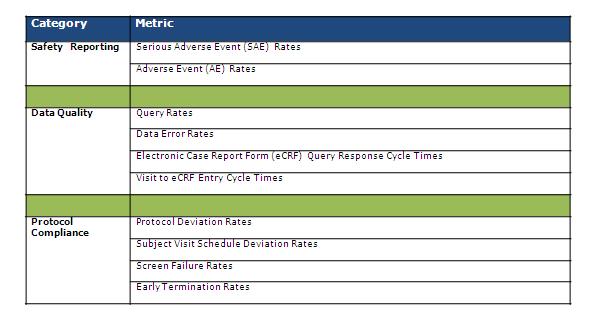
Figure 1: Key metrics for managing site quality
Measures like these can be computed for each participating site and for the study overall, using operational data typically captured in EDC systems. Unusually high deviations from an observed study benchmark can then be flagged and a “site quality risk level” assigned for each site for the given measure.
 ,
"The primary goals of this paradigm shift are to significantly improve the resource efficiency associated with clinical R&,D..."
 ,
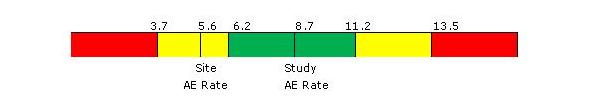
Figure 2 below depicts site quality risk level ranges for the AE rate on a hypothetical study. In this example, the overall AE rate for the study is 8.7 AEs per subject-year. The given site in the study currently has an AE rate of 5.6 AEs per subject-year, which places it in the “yellow” or elevated quality risk level for this measure.
The intent of this approach is to alert study teams proactively to a potential quality issue related to each measure. In the example, it is important to note that the “yellow” and “red” risk levels reflect only potential issues — not necessarily actual issues — for a given site. They represent what we call, “observations of interest,” which likely warrant further review or discussion. Depending on the nature of the identified issue, potential follow-up actions may include the following:
• Increasing the percentage of SDV required at the site,
• Delivering additional guidance and training on specific aspects of the protocol,
• eCRFs or other study logistics, and
• Planning additional on-site visits to more closely monitor certain aspects of the site’s study conduct.
For an SQM solution to be successful, it is crucial that it provide dashboard views on site quality measures for study teams, in a turnkey manner. The solution should be ready for use at the very start of the study, delivering near real-time intelligence that proactively alerts teams to emerging site quality issues.
Conclusion
As the industry continues its inevitable move to a risk-based monitoring paradigm, organizations are looking to measure, modify and optimize these efforts. In addition to establishing a risk-based monitoring strategy and study-specific plans, adopting the right enabling tools — including an SQM solution — can significantly accelerate the adoption of risk-based monitoring, and has the potential to drastically impact both in-study decision making and cross-portfolio planning.
About the authors:
Joshua Pines
Joshua Pines is a Senior Product Marketing Manager at Medidata Solutions Worldwide, responsible for Medidata Insights – a SaaS business analytics solution for clinical trial sponsors. Previously, he led software and life science marketing for Gerson Lehrman Group, the world’s leading expert network. Joshua has also held strategy and marketing positions with both Deloitte Consulting and IBM Software Group. In addition to extensive startup experience in software and new media, he has an MBA from the University of Miami and a BA in Psychology from Brandeis University.
Email: jpines@mdsol.com
Stephen Young
Stephen Young is Senior Product Director at Medidata Solutions Worldwide. Stephen brings more than 15 years of experience in driving change and implementing technologies within clinical development organizations, most recently as head of eClinical Services for J&,J Global Clinical Operations. In his current role, Stephen leads Medidata’s, Visualization, Analytics and Reporting portfolio of products, which includes Medidata InsightsTM, a SaaS business analytics solution providing customers with key metrics and analytics across the clinical research process. Stephen received his BSc in Mathematics from St. Joseph’s University and his MA in Mathematics from Villanova University.
Email: syoung@mdsol.com
How should your organization proceed with implementing a risk-based site monitoring strategy?