Pharmacovigilance and risk management information in centralised applications in the EU

Véronique Basch provides us with an overview of the new European Union pharmacovigilance legislation and how it affects the pharmaceutical industry. She also shares a timeline of the regulatory process.
Getting a new medication approved for the EU market through the centralised procedure can be complex and needs expertise. The new EU pharmacovigilance legislation (Good Vigilance Practice, GVP) is now in effect and applicants need to be fully compliant. It is critical to have a thorough understanding of the regulatory process and requirements, and the expectations with regards to the pharmacovigilance system and the risk management plan. Ensuring consistency and an appropriate level of detail in the documentation submitted as part of the approval process will help prevent unnecessary delays.
The process
The European Medicines Agency (EMA) is responsible for the centralised procedure and approval results in a single marketing authorisation. The centralised process is required for:
• Human medicines for the treatment of HIV / AIDS, cancer, diabetes, neurodegenerative diseases, auto-immune and other immune dysfunctions, and viral diseases;
• Veterinary medicines for use as growth or yield enhancers;
• Medicines derived from biotechnology processes, such as genetic engineering;
• Advanced-therapy medicines;
• Orphan medicines.
For medicines that do not fall within these categories, companies have the option of submitting an application for a centralised marketing authorisation as long as the medicine concerned is a significant therapeutic, scientific or technical innovation, or if its authorisation would be in the interest of public or animal health.
"It is critical to have a thorough understanding of the regulatory process and requirements..."
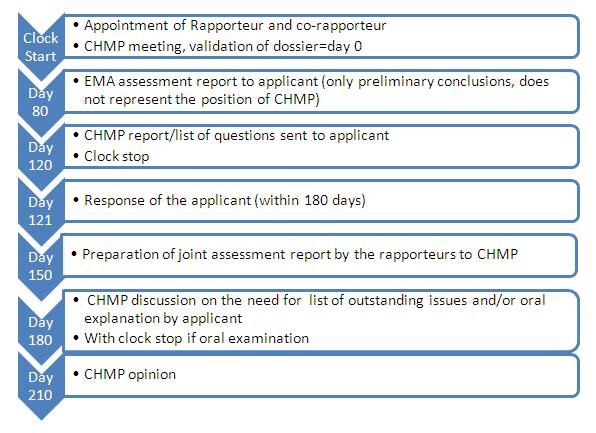
EMA's evaluation takes up to 210 days and the recommendation is transmitted to the European Commission. The process includes 2 potential clock stops, where the applicant is provided with questions to answer on the application content. Indeed, the fewer the questions, the earlier the applicant will be able to respond and restart the clock. A good anticipation of potential questions will ensure an earlier approval.
Figure 1: Timeline showing the steps for submitting a centralised marketing authorisation application
Requirements in safety related data or documents
An application for a centralised marketing authorisation requires several safety-related documents including:
• A summary of pharmacovigilance systems, which replaces the detailed description of pharmacovigilance systems
• A risk management plan including namely :
o Safety Specifications
o Pharmacovigilance Plan
o Plan for Post Authorisation Studies
o Risk Minimisation Measures
Safety concerns from clinical and non-clinical studies are summarized in different documents (SmPC, RMP, CTD sections 2.4, 2.5) and consistency among these is important. This can be challenging because they are often prepared by different teams.
Risk management plan
When building a risk management plan for a centralised procedure application, it is important to find the right balance in offering risk minimisation or surveillance activities. Offering too much to health authorities initially means there is nothing left to negotiate and the manufacturer expends more resources than necessary. Contrarily, offering too little could result in substantial delays, loss of credibility and imposition of additional activities.
"...it is important to find the right balance in offering risk minimisation or surveillance activities."
Therefore, it is important to identify and classify the risks appropriately. The way these are selected and described raise a lot of questions at the authorities, if not done appropriately. Always remember:
• Important (identified or potential) risks can have an impact on the benefit-risk balance of the product. These typically include "any risk that is likely to be included in the contraindications or warnings and precautions section of the product information should be considered important."
• Potential risks may be derived from non-clinical studies, pharmacological class effects or arising from clinical trials and signal detection (causal association not confirmed).
• Important missing information (populations not studied during development) must be described.
Some examples of topics considered as missing information includes:
• A cautionary statement about use of alcohol for a drug that has a hepatotoxic effect should be included in important missing information, until post-marketing studies demonstrate that there is no risk.
• Information about populations in which the drug was not studied, such as non-Caucasian populations, should be included.
• Missing information concerning treatment in pregnant and breast-feeding women, children and elderly should be included.
It also is important for manufacturers to suggest appropriate actions for the management of the risks and take risk minimisation measures. This can include routine measures such as inclusion in the Summary of Product Characteristics, package labeling, Patient Information Leaflet, pack size and design and prescription status of the product. Manufacturers should also prepare for additional risk minimisation activities and evaluation of such. Additionally, the applicant may be required to run a post-authorization safety study (PASS), which may take many different forms, as defined in the new EU GVP.
"For manufacturers looking to launch a new product in the EU market, these are key points to keep in mind."
For manufacturers looking to launch a new product in the EU market, these are key points to keep in mind. Proper preparation, coordination and consistency in putting a centralised procedure application can help ensure approval in the optimal timeframe.
There should be thorough consultation with internal and external stakeholders and when unsure of any elements of the process, pharmaceutical manufacturers should seek expert advice from companies that have expertise in optimizing EU applications for new drugs.
About the author:
Véronique Basch, PharmD
Executive Director, Global Pharmacovigilance, UBC
Véronique Basch, PharmD, serves as the Executive Director of Global Pharmacovigilance. In this role, she manages the development, implementation, and execution of UBC's safety strategy, providing direction, oversight, and management of its global Pharmacovigilance TeamVéronique first gained international research experience in California and later at the University Hospital of Zürich. She has experience in all aspects of pharmacovigilance and drug safety, including data management, training, medical analysis, periodic safety update report (PSUR) writing, quality assurance, project management, and team management. Véronique holds a PharmD from the University Louis Pasteur in Strasbourg, France and later graduated from the University of Basel as a University Professional in Pharmaceutical Medicine.
In what ways will the new EU pharmacovigilance legislation benefit pharma manufacturers?