News

Ipsen crosses EU finish line with Alagille syndrome drug
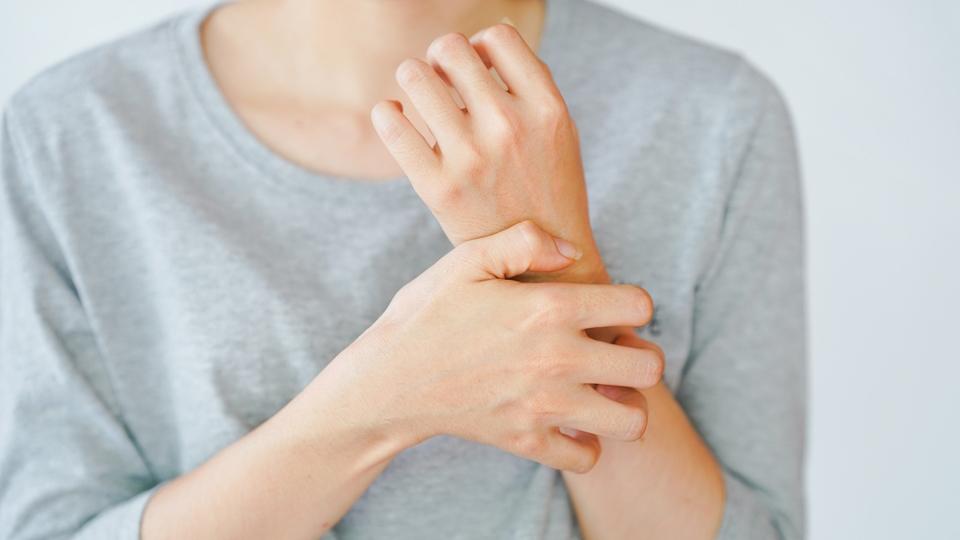
After various twists and turns in the regulator path, Ipsen has secured EU approval for Kayfanda, its treatment for severe itching (pruritus) in patients with the rare liver disease Alagill