12 Questions with Pilar de la Rocha Mur

Pilar de la Rocha Mur is Head of Clinical Operations EMEA (Europe, Middle East, and Africa) at BeiGene. She brings over two decades of experience in Oncology Clinical Development, starting with translational work at UCLA and later overseeing the Early Clinical Development Cancer Center at Harvard Medical School in DFCI, Boston. Her journey continued with leadership roles in global Clinical Oncology programmes at Novartis in Switzerland, extending to the Asia Pacific & South Africa region, based in Singapore, leading the execution of clinical trials of all phases in over 20 countries. During her last seven years at Novartis, De la Rocha Mur led the Operational Excellence team in Translational Clinical Oncology, contributing to streamlined processes and high-quality trials, and designed internet-based platforms streamlining activities related to site management, feasibility assessment, and patient enrolment. Joining BeiGene in mid-2022 as the Global Head of Operational Excellence, De la Rocha Mur initiated impactful changes in Clinical Operations, fostering compliance and efficiency, and in early 2024, she transitioned to the role of Head of Clinical Operations EMEA. De la Rocha Mur is dedicated to building a robust and compliant organisation to better serve patients in Europe. Based in Basel, Switzerland, she is a devoted mother, a yoga teacher, and an outdoor enthusiast. Her interests include walking in the forest, skiing, swimming, and regular yoga practice. De la Rocha Mur's passion for travel allows her to connect with family and friends, rounding out a fulfilling professional and personal life.
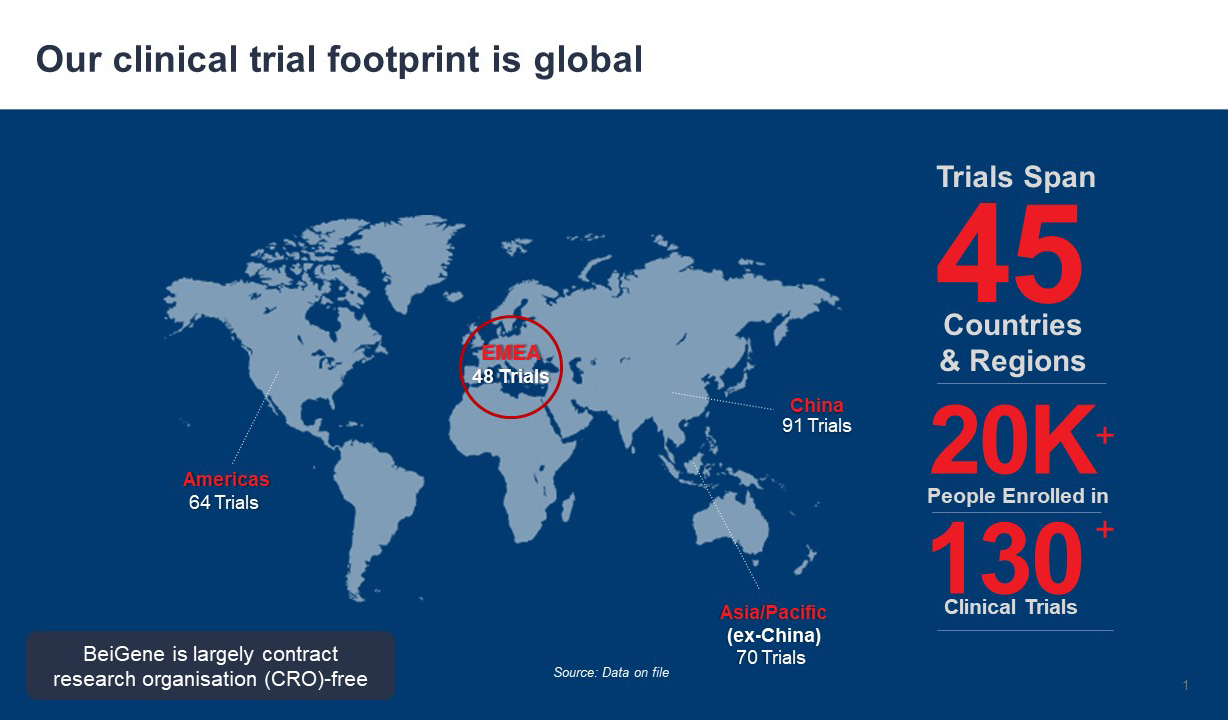
What are the main responsibilities of your current role? My role at BeiGene is to expand clinical trial reach across Europe, the Middle East, and South Africa by accelerating contributions to global trials and increasing available drugs for patients. We aim to optimise our clinical footprint by strengthening site networks in key territories.
What is your background prior to this role and how did it prepare you for the work you do now? In August 2022, I joined BeiGene as Head of Operational Excellence, leading global functions including Compliance, Inspection Readiness, Process Transformation, Learning and Development, eTMF, Record Management, and Post-Trial Supply. This role provided insights into the biotech landscape, informing my approach to success and improvement in my new role.
What is your proudest professional accomplishment to date? During my early days at the University of California, Los Angeles, in 2004, I worked in the Haematology-Oncology department's research laboratory. There, we treated a stage IV melanoma patient with a ‘homemade’ anti-melanoma therapy and observed a clinical response: a moment where I realised that everything we were doing in the lab was helping the patients get better. Every piece of clinical research we do at BeiGene is an action towards helping patients in need.
Since then, I’ve supported the development of over 100 new drugs, some now available in the market for the treatment of several tumour types.
Now 20 years later at BeiGene, I am proud to guide and mentor clinical research professionals who share my passion for advancing affordable cancer treatments.
What are some of the biggest ongoing challenges in your work? The core challenge is always the next discovery. We remain vigilant, asking, “what unmet needs do patients face?” and striving to develop treatments accordingly. In the EU, 2.7 million people are diagnosed with cancer every year, a number that continues to grow. At BeiGene, we aim to bring multiple high-quality new molecules from discovery into the clinic each year, focusing on potential first-in-class and best-in-class candidates in early clinical development. We advance the most promising options in later-stage trials, always anticipating the next challenge.
My primary goal is to drive progress in discovering new treatments, while ensuring equitable access to innovative medicines across Europe, the Middle East, and South Africa - a core value at BeiGene. All our efforts to develop new, innovative medicines are only worthwhile if they reach the patients they were designed to help.
What is your personal mission statement? What values keep you centred in your work? In both my professional and personal life, I prioritise hope and possibility over fear, focusing on what should happen, rather than what should not. I share the urgency felt in this field, recognising the daily diagnosis of new patients who may benefit from novel therapies. The direct impact of our work on society motivates me deeply.
What are your biggest short-term goals for this year and next year? This year, our focus is on building a strong clinical operations infrastructure across our territories to solidify our presence in EMEA. We’ll establish new collaborations with key clinical sites, collaborative groups, and their experts in oncology, advancing therapies for haematology and solid tumours.
By 2025, we’ll be ready to manage a diverse pipeline of new molecular entities and engage in large registrational studies, expanding patient access to treatments. We’ll also explore opportunity for expansion in other territories, acknowledging healthcare diversity and access limitations in some countries.
What are the most professional skills in your work and how do you hone them? In this role, strong drive is essential, as finding cancer cures is urgent; patients are dying every day, demanding relentless effort. We must maintain focus on the big picture in clinical research.
Discipline is paramount due to heavy pharmaceutical regulations. We must uphold high standards, generate data efficiently, and adapt to regulatory changes. Collaboration is key for R&D innovation, both internally and externally. Strategic alliances with pharmaceutical firms and academic institutions drive innovation. When conducting trials, companies rely on medical community expertise and hospital staff to collect patient data for regulatory submissions. Overcoming global health challenges like cancer requires collective effort.
What excites you most about current industry trends? I’m particularly excited about current industry trends that promise to enhance our R&D capabilities significantly. Developing new medicines is highly R&D-intensive, with about 90% of investment allocated to clinical trials. This investment covers the costs of establishing investigator centres, treating patients, and evaluating results.
Investing in in-house research capabilities and clinical development, including personnel and technology, accelerates the discovery and development of new medicines. This approach offers greater control over trial quality, speed, and efficiency, fostering increased engagement with site investigators.
At BeiGene, we’ve established our own fully integrated infrastructure, with a global clinical development and medical affairs team of over 3,000 people conducting the majority of our global clinical trials. This is central to developing affordable medicines.
How do you promote patient centricity in your workplace? As Head of Clinical Operations in EMEA, promoting patient centricity is a cornerstone to our approach to health equity. Patient-centric principles permeate every facet of our operations, beginning with trial design. BeiGene collaborates with the patient community throughout the drug development journey, from scientific discovery to clinical trials to regulatory submissions, in order to ensure that their perspectives and needs are central to our study protocols and to the medicines we bring to market. Diverse participation in clinical trials is essential to advancing health equity, enhancing data robustness, and accurately representing the patients our medicines aim to serve once approved.
What advice would you give to a young person starting out in your field? From a Clinical Operations perspective, I would say: “Get out there and get exposed to as many angles of clinical research as you can!” Success here is directly related to the diversity in experience and exposure you have. There are so many different aspects of clinical research and each of them offers invaluable insights.
My other piece of advice is: “Keep your foot on the (gas) pedal! And never work in a rush”. Maintain high-quality standards in everything you do, so we can ensure that the safety, wellbeing, and rights of the patients are always protected.
What are your hobbies? What do you do in your free time? I cherish my free time, often spent with my seven-year-old daughter and my dog. We like outdoor adventures, exploring new places, especially in Switzerland.
Whether skiing in the Alps or swimming in the Costa Brava, Spain, I revel in nature’s beauty.
How do you manage health, fitness, and wellness in your life? Prioritising health and well-being is vital for peak performance, both personally and professionally. I maintain a clean, Mediterranean diet and prioritise quality sleep. Daily yoga practice, including teaching online classes, enhances my physical, emotional, and mental wellness.

Connect with Pilar de la Rocha Mur on LinkedIn.












