Advancing innovation in life sciences R&D and Quality: Towards a single source of truth

This year’s Veeva R&D and Quality Summit saw full-house attendance at the Marriott Auditorium Hotel in Madrid. The summit, brought together 1,200 industry leaders to explore how to enable faster trials, higher data accuracy, and advanced patient safety, enabled through software, data, and now business consulting – all as delivered by Veeva.
Do more and go faster
With Accenture, Base Life Science, NNIT, and Translations.com as this year’s platinum sponsors, and gold sponsors including Cognizant, PwC, and Syneos Health, it was Jim Reilly, VP of development cloud strategy at Veeva, who presented the company’s current and intended capabilities for scale.
Do more and go faster, he said, without adding people in a linear fashion to organisations. It’s about more than technology, even. People, process, technology – these are all aspects in Reilly’s own background and at Veeva also work as a triple aspect when it comes to industry expertise, deep product knowledge, and data-powered insights. With over 50 programmes in business consulting in 2024 (up from a mere one only last year), the company is driving change within organisations globally, including in business process optimisation, change management, and value realisation.
That buzzword AI
And then, of course, there’s AI. Artificial intelligence has taken over the mindshare of the industry, and rightly so, said Reilly. Still in a hype cycle, but delivering value, industry is still in the early stage of experimenting with AI, while also proving out use cases and expanding on them. During this process, two things are becoming clear: GenAI will become a competitive differentiator for individual companies, and there will be preferences for assistance from these technologies.
The Direct Data API is a new class of API that provides high-speed read-only data access to Vault. Designed to be used to replicate large amounts of Vault data to an external database, data warehouse, or data lake, it can be employed for analytics on extracted data residing in a data warehouse, as an integration hub, and for choosing data to train LLMs with. Meanwhile, via the AI Partner Program, data can be extracted quickly through the Direct Data API, for building innovative GenAI solutions and delivering results and data right back into Vault, where customers can incorporate them directly into their workflows and business processes.
AI and clinical transformation: High-value or hype?
Later in the Summit, Novo Nordisk shared its own strategy for transforming clinical research through the use of data and AI. The clinical adoption track was introduced by Hugo Cervantes, VP of Vault Clinical Strategy at Veeva, and Richard Young, VP of Clinical Data at Veeva introduced Thomas Senderovitz, Novo Nordisk’s Senior Vice President of Data Science, to a packed room of over 300 people.
Senderovitz leads a team of 1,100 in data science across multiple disciplines. He said that there is no doubt that AI is a buzzword, but he asks that industry be specific about what it means when talking about AI. In his first slide, he ‘added on’ digital transformation: “to keep things grounded.” Additionally, preferred terminology for Senderovitz discards ‘patients’ for ‘people living with disease’. “It’s not ‘humans are bad and machines are good’,” he said.
In 2023, the Novo Nordisk portfolio included more than 55,000 patients across over 150 clinical trials across 6,100 active investigator sites in more than 60 countries. They have clinical trial activities in 10 therapy areas. Senderovitz explained that he’s not a good surfer, but even if he were, he wouldn’t want to surf a tsunami; however, he would like to know how to, if a tsunami were a possibility. And digital transformation is surfing that tsunami.
To this predicament, the company utilises a ‘5D approach’: data, drug, digital, device, diagnostics:
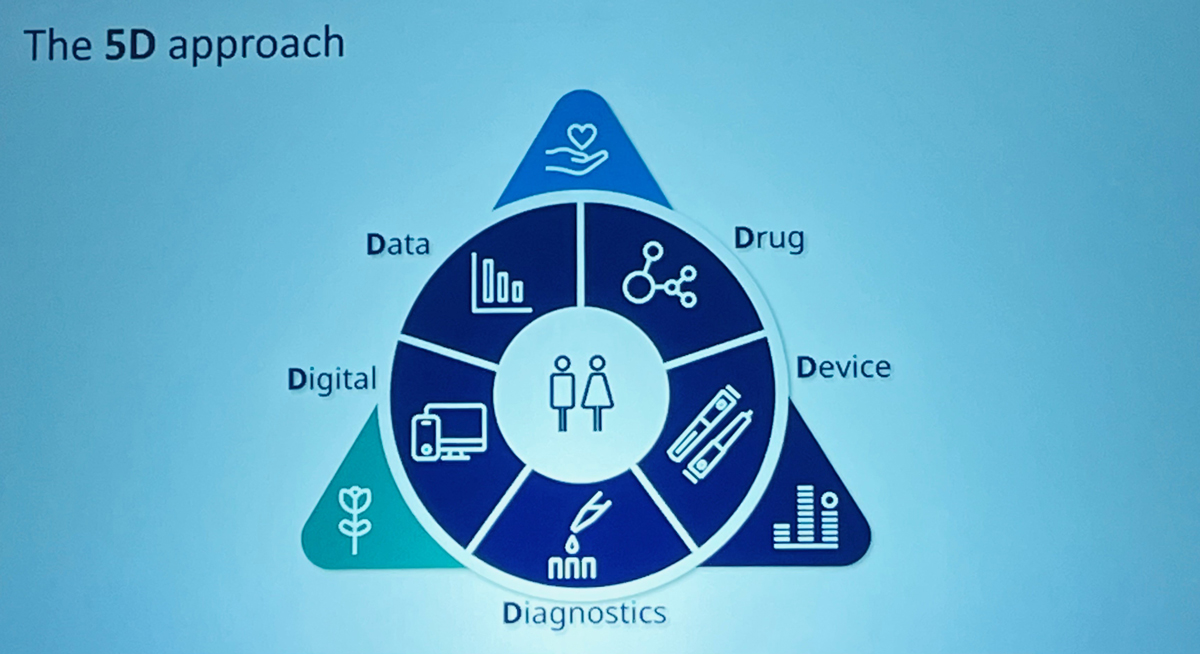
To be clear, Senderovitz himself “hates apps”, deeming them ‘yesterday’: “All models are wrong, but some are useful; most apps are useless, but some are useful. You just want to live a seamless life, you don’t want an app telling you want to do and what not to do,” he bemoaned.
Still, as a drug company, Novo Nordisk is nonetheless adding other elements into the mix, integrative treatment solutions. It’s considering socioeconomic factors, food and drink, and multiple lifestyle considerations. Enormous amounts of data, then.
The reality right now is a growing complexity in clinical trials. Things must be done at scale, not just for citizens, but for clinical set-up: scientific insights, risk reduction & quality, scalability & productivity, speed (cycle time). In other words, quality by design, algorithms with insights and identification. About 15 years ago, all of this just wasn’t possible.
Four pillars central for growth at Novo Nordisk are a strengthened data foundation, innovative study programmes, automation, and ecosystem and partnerships. AI is all good and well, but you need access to high quality data, said Senderovitz. Automation is not necessarily “a cruel VP who wants to get rid of his colleagues - not all of them.” Rather, it’s about speeding up processes. Nevertheless, automation has to be done in a way that adds most value. To this end – another buzzword – ‘partnerships’ really are vital; all this cannot be done in isolation.
What COVID-19 taught was that it’s not always necessary for patients to come into clinics for treatment and the like. Additionally, Senderovitz is a firm believer that simulations should be a prerequisite before running clinical trials.
“We’re all human beings – [or] predominantly human beings in data science. [We’re] not looking for perfection, but high ambition,” concluded Senderovitz.
A matter of safety and clinical
Rik Van Mol, Veeva Systems’ SVP of Development Cloud, and Avril England, Veeva Systems’ general manager of Vault, began with Vault Safety in their overview presentation at the Summit’s start.
Safety is yet an area in need of innovation – a fact which a later UCB and LEO Pharma panel attested . Legacy systems are not really evolving. Nonetheless, global case intake and processing across the globe – safety in the cloud, in other words – is able to work at scale. Risk management will be incorporated later this year to support the safety processes in a seamless and integrated solution with SafetyDocs. Equally important, as mentioned, is AI and advanced automation.
Under the Veeva Clinical Platform – which includes MyVeeva, eConsent, an ePRO Survey, Study Information, and Appointments – improvement in clinical research is being facilitated with free software and global support to sites. Over 7,500 with site vaults exist today, and each login uses Veeva ID, a single ID sites can use to access the technology. At the end of the day, the goal is site simplicity.
Veeva Clinical Operations, the foundation for trial execution, has more than 10,000 connected study sites using Site Connect A. It includes study start-up, eTMF, CTMS, payments, and study training on a single cloud platform, so as to accelerate trial execution and deliver real-time visibility. The aim is to eliminate systems silos, streamline end-to-end clinical trial processes, and improve collaboration for sponsors, CROs, and clinical research centres.
Additionally, there are two applications coming up: Vault Disclosures for management of publicly sharing clinical trials results as required by the authorities, planned for December this year in Europe, managing EU CTR and data requirements; and Veeva OpenData Clinical, reference data for clinical operations, for both investigators and sites, for the purpose of eliminating duplicative data and increasing reporting accuracy.
An industry case study: Bayer
But what of industry perspective, rather than Veeva itself? Well, Bayer’s Emma Earl, head of clinical trial management services and solutions, took to the stage to discuss the pain of integrations without an integrated platform, a scenario with which many in the room were familiar with, data taking time to get from A to B. Then, there are reviewing processes as part of implementation projects, looking at whether applications or integrations can be eliminated. What Earl also realised was just how poor the quality of emails as a data system really is…
 Now, they’re working across approximately 40 countries, with around 45 sites – with different states of comfort with the implementation of such technology. Everyone present at the summit, in fact, exists at a different comfort level with these technologies (some, indeed, still want to hold onto paper notepads). Those sites that Bayer can see moving ahead are propelled by wanting to relieve administrative burden, getting treatments to patients faster.
Now, they’re working across approximately 40 countries, with around 45 sites – with different states of comfort with the implementation of such technology. Everyone present at the summit, in fact, exists at a different comfort level with these technologies (some, indeed, still want to hold onto paper notepads). Those sites that Bayer can see moving ahead are propelled by wanting to relieve administrative burden, getting treatments to patients faster.
There has, however, also been negative feedback, but Earl thinks the next general release coming from Veeva will address those concerns, here alluding to the outages that affected the system at the end of April. On this point, Avril England later reasoned that there are almost four million active users of Veeva Vaults globally today, with over 600 million documents stored and available, and it takes under two seconds to view each document. With over 16 billion data records globally, relentless focus on the space has led to this point. However, making changes to the technology and processes, based on its commitment to its customers for decades to come, brings new demands to the system. In order to ensure its operational excellence in future, Veeva now provides a 10-minute upgrade to 97% of its users (down from six hours only a few years ago). This is, it is fair to say, impressive, given also that a year ago that figure was 75%. In England’s words, “10-mins is a cup of coffee.”
And it is deep partnership that makes all this happen.
Trends and TMF Bots
Meanwhile, as reported by ProPharma, the Trial Master File (TMF) allows an effective way to collect and manage study specific documents during a clinical trial, serving as a complete and accurate record of the trial's conduct, including protocol, informed consent forms, study reports, and other documents of import.
Given the volume increase of documents from clinical trials today, manual management can be a mammoth task. To this end, the TMF Trend Report for 2024, resulting from the Veeva TMF Innovation Forum, provided seven trends in TMF: automation, study health scores, ICH E6(R3), inspections, outsourcing, data standardisation, and M&A.
In June last year, it was reported that over 450 biopharma companies use Veeva Vault eTMF to automate trial processes and improve information exchange across stakeholders, more companies seeking to increase transparency and inspection readiness. This includes the TMF Bot to automate document classification for faster, more efficient processing and improved accuracy (the TMF Bot has classified more than one million documents). TMF Bot is an AI-driven solution within Veeva Vault that employs natural language processing (NLP) and machine learning (ML) algorithms to automate categorisation of documents within an electronic TMF.
An industry case study: Jazz Pharmaceuticals
 Diane Black, Jazz Pharmaceuticals’ SVP Global Quality (Chief Quality Officer) stated how hers is a slightly interesting organisation, in that it has historically grown by acquisition, most recently with the acquisition of GW Pharma in 2021, and uses Vault Quality Docs as a single organisation – there is no ‘This legacy this, this legacy that’. The key benefit of this process, she says, is a single source of truth; there is only one version.
Diane Black, Jazz Pharmaceuticals’ SVP Global Quality (Chief Quality Officer) stated how hers is a slightly interesting organisation, in that it has historically grown by acquisition, most recently with the acquisition of GW Pharma in 2021, and uses Vault Quality Docs as a single organisation – there is no ‘This legacy this, this legacy that’. The key benefit of this process, she says, is a single source of truth; there is only one version.
People are attached to their Quality processes for some bizarre reason, said Black, but advised, “Don’t try to boil the ocean.”
On improvements still needed, within her ‘laundry list’ were CDMO data, the AI space (simple automation and GenAI), connecting vaults (RIM with QMS, LIMS with QMS), and getting better at the continuous improvement cycle.
UCB and LEO Pharma: Making the case for transformation with digital pharmacovigilance (PV)
A panel with UCB and LEO Pharma later during the Summit explored how rapid technological advancements are making a compelling case for transformation. Discussing simplifying and automating pharmacovigilance (PV) and strategically leveraging data for deeper insights, the panel included Pilar Carrero, LEO Pharma’s head of safety and VP of R&D process optimisation; Adrian Maynier, UCB’s head of systems in patient safety; Jonas Maselis, UCB’s head of IT patient safety; and was moderated by David Kolosic, Veeva Systems’ senior manager of Vault safety.

A lot can happen in a few years, said Maynier, and the story for UCB has been one of patients in that time. Identifying Veeva as a partner across the portfolio of the company’s R&D, Veeva has had a strong role to play when it comes to safety. The question for UCB has been, ‘How and when?’
Maselis is ex-clinical coming into safety, by contrast. His adventure only beginning in February, joining PV from clinical, in this new space, it is clear that “there is time to do things right” in this space. It is, perhaps, an outside perspective and a fresh view on things that have permitted him the mindset that there is a comfort in this space, a stage-defined approach with clear objectives – and it is the right track to be on.
Conversely, LEO Pharma’s Carrero, on her drivers for change, noted the company’s recovery from a failed implementation with a different vendor. It is, thus, an ‘obvious choice’ to have Veeva for safety.
What is the vision for safety as a whole and why must that strategy be defined from the beginning before embarking on the journey, though, asked Kolosic?
Maynier said safety’s vision has to fit into that of R&D at UCB. Their five key objectives are compliance, productivity enhancement through automation, productivity through process change and organisation, augmentation (the improvement of data quality and transparency around signal tracking), and insight generation. So it is that the Veeva Workbench is coming at the right time for them.
For Carrero, the vision is the same: compliance is something that simply must be done, but also has a lot more to offer for an organisation. That agenda, however, needs to be pushed: what is the value being brought? It is technology that can help in realising this. Now is the time to grab opportunities, get out there with the rest of the company and sit at the important tables, alongside a listening Commercial team.
The benefits are not seen in HQ, Maynier noted, on a country level, eliminating multiple local locks and doing away with the need to reconcile. In general, people feel much more on top of their work, he said, which benefits compliance, and the journey continues in that respect.
It is, again, about one source of truth, said Carrero: “Let’s utilise the data where it sits, rather than multiplying it in many different places. [We] don’t need to eat the elephant at once; move pieces bit by bit.”
Maselis, remembering preparing for his interview process internally at UCB, checked out the infrastructure and noted a ‘spiderweb’: streamlining was critical. Not building an Elon Musk space shuttle, it is, he said, rather just a matter of the technology layer.
With volumes and complexities going up, it’s no longer a case of simple algorithms - how can one confidently answer inspections with these technologies? asked Kolosic.
Inspections are increasingly data oriented, said Maynier, and being able to report and provide analytics quickly and efficiency is crucial.
Meanwhile, Maselis added the criticality of data connection. If you can compare any data, through different forms and systems, he said, noticing specific data – for example, tracking your pizza delivery – at any point in time, you should be able to know the specific status. Industry can advance yet on this, and might be at that point in time with GenAI.
Carrero noted an inspection last year, and this matter of traceability. Having a unified platform makes it so very much easier to follow. In that particular inspection, the need for reconciliation and logs was eliminated, surprising the inspector, and they had to explain why that was, due to the platform…
On the need for a foundation, for clean high-quality data that can be trusted, Maselis commented that it is a healthy ambition and the will of people to change that matter.
Crawl, walk, run, eventually even fly maybe – where can standardisation be done better? asked Kolosic.
There’s a hit list, according to Maynier: affiliate processes, and follow up activities in case operations, in terms of standards, as every country has their own. Secondly, signal management processes – getting terminology and data out there.
Carrero agreed in respect of affiliates and noted how everyone working the same system facilitates matters, facilitates the conversation with tangible content. Thus LEO Pharma has standardised archiving and follow up. What remains is a need for patience, however.
The pharma world is complicated enough; it is a complex world that has been further complicated on top of its own complexities by industry itself, stated Carrero. It’s a matter of fighting that history.












