How a clinical metadata repository can help with data quality

Metadata comes from multiple sources and can be stored in different places, systems, and networks. It’s hard to track it down. When files are updated, new versions are created. So how do you know if you’ve got the latest version? How do you know if the quality is good enough?
The easiest way is to use a clinical metadata repository! And, by creating organizational standards that adhere to industry standards, data will be reliable and consistent. You’ll also have greater transparency.
What’s a clinical metadata repository?
A cloud-based clinical metadata repository is essentially a database that maintains metadata definitions such as forms, datasets, codelists, and variables, throughout the various stages in a clinical trial.
Metadata plays an essential role in allowing different people involved in a clinical trial to access, monitor, track, and log data. All your teams can access information in a readable format, easily and quickly. And, allows for effective planning, communication, and teamwork.
It gives total transparency to all users and ensures that data is of a high standard. Both current and historical metadata should be accurate and easily accessible.
A clinical metadata repository is key to effectively managing organizational standards. It lets you:
- Create, maintain, govern, and use standards consistently.
- Reuse your existing assets.
- Realize the impact of changes.
- Create accurate mappings.
- Be fully compliant.
- Create high-quality submissions.
There are various features that contribute to data quality.
Governance
You can create your own organizational lifecycle for studies and standards to transition through according to your company’s governance process.
Aside from improving data quality, governance lets you control and fully understand the workflow and develop robust organizational standards. This means you can get your product to market safely, and faster.
If metadata isn’t properly managed, it can become out of date and invalid. Good governance means your metadata is accurate and compliant.
Reuse
Organizational standards are stored ‘all in 1 place’ and can be reused. For example, forms, mappings, annotations, controlled terminology, and datasets. A standard can then be updated to suit study-specific requirements. Outputs can also be automated. And, because standards have already been approved, tested, and validated, it means data quality is improved and remains consistent.
Impact analysis
One of the key objectives is to analyze the impact of change. All associated standards and assets will be analyzed to let you know exactly what downstream or upstream metadata will be affected. Impact analysis should also show all assets that are indirectly affected. You can also see how your assets interrelate in the metadata repository. The diagram below shows how the CRF can be affected by a change in the ADaM dataset.

You can also see how your assets interrelate in the metadata repository.
Impact analysis lets you make informed decisions before you make changes. You know the scope of the updates. And, once you have this information, you can decide whether it’s worth making a particular change or not.
Change control
Team members can set up change requests to change existing standard objects. For example, updating a form. The change control process is a pre-defined workflow that defines the approval process as well as the tracking and handling of change requests. All changes are tracked from inception to completion.
Example of an approval process:
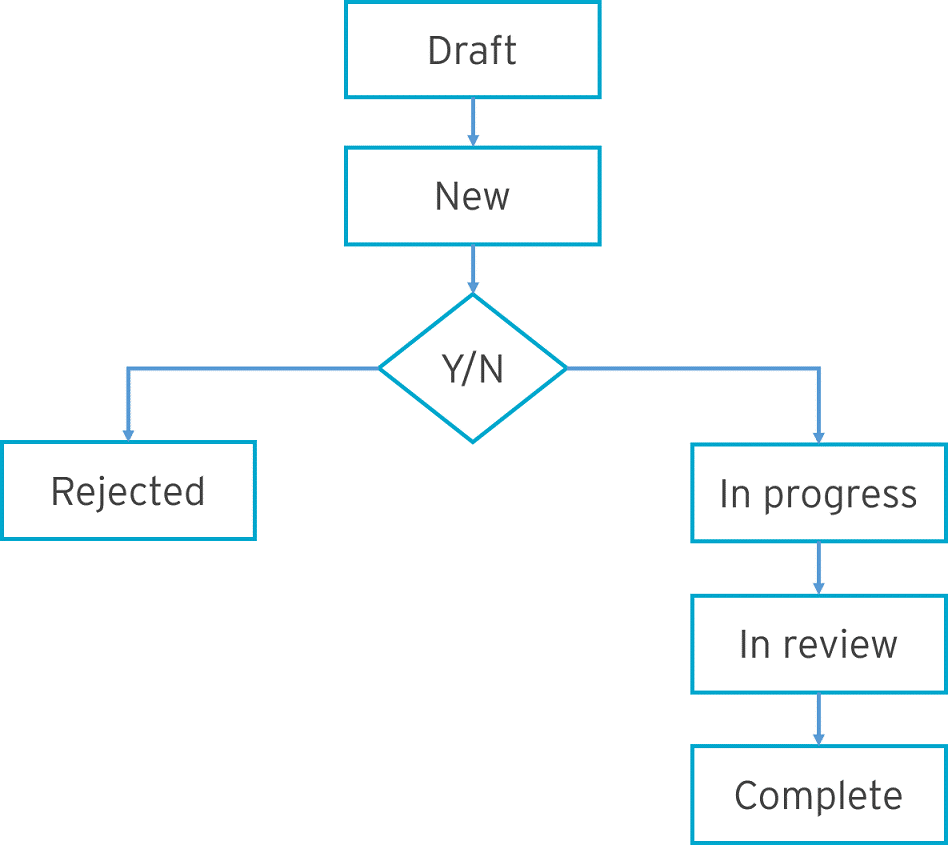
Versioning
A good clinical metadata repository allows multiple versions of the same standard that has been updated, improved, or customized.
You can easily identify which version of a standard is being used. And, users can be confident they’re working on the correct version of an asset or standard.
Traceability
Traceability is of key importance in the world of clinical trials, due to the ever-changing regulatory environment.
Traceability must be built into a clinical metadata repository so that all assets can be fully tracked through their lifecycle. With traceability in place, you can see who has accessed the clinical metadata repository. Who made changes to what studies, standards, and assets, and when. And, you can check the differences between them. For example, the differences between versions of the same standard. You can see the full and detailed history of a standard.
Full traceability throughout the lifecycle process ensures audit compliance and increases the chances of a successful submission to the FDA.
Conclusion
The real measure of data quality comes at submission time. Are many questions raised? And, how long does it take to resolve them? If the answer is “not many” and “not long”, then you know without a doubt that the quality of your data is high.
Can Formedix help?
Our clinical metadata repository and study automation platform has been built especially for clinical metadata. It’s off the shelf which means you can get started straight away! It covers all the data quality aspects discussed in this blog.
And, we’re constantly developing it in line with what’s happening in the industry and with the latest standards and regulations.
The Formedix platform is used by many pharma companies, biotechs, and CROs. Each organization has its own objectives and processes, and we work with customers to meet their individual needs. Common goals include:
- Developing internal standards that can be reused.
- Having a central place (MDR) to store forms, datasets, standards, and other study data/metadata.
- eCRF design (in Rave, InForm, or another EDC).
- EDC specification.
- EDC build.
- Quickly creating define.xml from SDTM datasets (automate SDTM conversions).
- Getting ADaM datasets into define.xml.
- SDTM validation.
- Creating Analysis Result Metadata in define.xml.
- Automating end to end studies from eCRF through to submission.
- Seeing data much faster.
- Easier clinical metadata management.
Any of these sound familiar?
You can request a no-obligation demo to see how our automation platform could help you. Or arrange a call so we can talk through your situation and go from there.











