UK experimental drugs bill 'could hit clinical trial recruitment'

Patients in England and Wales will be able to get access to experimental medicines from next March if a new bill is adopted - but there are fears the measures could make it harder to test new drugs.
The Medical Innovation Bill - which was proposed by Conservative peer Lord Saatchi after his wife Josephine Hart died of ovarian cancer - is designed to allow terminally-ill patients access to untested drugs, and also to protect doctors who prescribe them from any legal action if the treatment fails or causes unexpected side effects.
It has polarised opinion in the medical profession and among patient groups, and had to be re-drafted several times. The Bill now looks increasingly likely to become law, although opponents seem to be outweighing supporters on Twitter (#saatchibill).
Various changes - such as requiring doctors to get agreement from a another specialist before prescribing an experimental therapy - mean that Health Secretary Jeremy Hunt is now backing the Saatchi bill and even former opponents such as the General Medical Council (GMC) are voicing guarded support.
The scope of the bill has also been dramatically reduced, and is now restricted to cancers that would otherwise result in the patient's death or other cases specifically sanctioned by the Secretary of State.
It would sit alongside other measures such as the Early Access to Medicines scheme, unveiled by Hunt earlier this year, that will allow unlicensed treatments to be prescribed to seriously-ill patients providing they have shown promise in clinical trials.

Ana Nicholls, a healthcare analyst at the Economist Intelligence Unit, notes that the changes have brought the bill closer into alignment with the World Health Organization (WHO) and US federal guidelines that have been implemented to allow unproven Ebola treatments such as Mapp Biopharmaceuticals ZMapp to be used in patients.
While this should help to allay many of the concerns raised by previous versions of the bill, Nicholls questions the effect on formal clinical trials for advanced cancer treatments.
"At present, patients who want access to experimental treatments and are willing to take the risks usually sign up for clinical trial," she says, pointing out that around 600,000 people opted to participate in UK trials last year.
"Such trials rely on some patients receiving a placebo instead," she continues. "If they can persuade their doctors to give them experimental treatments directly, then trial recruitment may drop."
That said, the numbers willing to enrol in studies suggests the avenue opened up by the Saatchi bill could be very popular, adds Nicholls. The numbers "suggest that the demand for experimental medicines is very high, and that those facing death from cancer are likely to welcome the new bill."
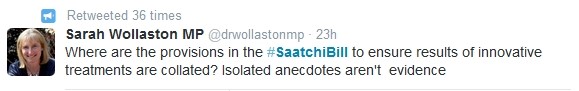
Others are concerned that the bill does not contain any mechanism to ensure that data is collected and disseminated when patients get access to experimental therapies, a principle which underpinned the WHO's recent deliberations on the ethics of providing untested Ebola therapies to patients.
The Saatchi bill does have vocal opposition - including clinical negligence lawyer Nigel Poole of Kings Chambers - who argues that existing guidance from the GMC provides an opportunity for patients to access innovative treatment if a "responsible body of medical opinion" support it, so is unnecessary and could help protect doctors in cases where negligence is suspected.

"In response to concerns that the bill would expose vulnerable patients to harm from maverick doctors, the amendments now proposed set out a labyrinth of new procedural requirements," he argues in a recent blog.
"So unclear are these requirements that they are highly likely to lead to more litigation and more red tape."
The proposals are scheduled to be debated in the House of Lords on Friday 24 October.










