TG Therapeutics cancer trial setback sends shares tumbling
TG Therapeutics (TG) has suffered a setback in its blood cancer drug trial, as the data available was deemed not ready for analysis.
The independent Data Safety Monitoring Board (DSMB) said that an interim analysis of the overall response rate (ORR) of TG’s UNITY-CLL phase 3 trial ‘could not be conducted at this time as the data were not sufficiently mature to conduct the analysis.’
This news sent New York City-based TG's shares tumbling by 30% in pre-market trading on the Nasdaq stock exchange.
TG’s trial compared the combination of ublituximab plus umbralisib, or U2, to an active control arm of obinutuzumab plus chlorambucil in patients with both treatment naïve and relapsed or refractory chronic lymphocytic leukaemia (CLL).
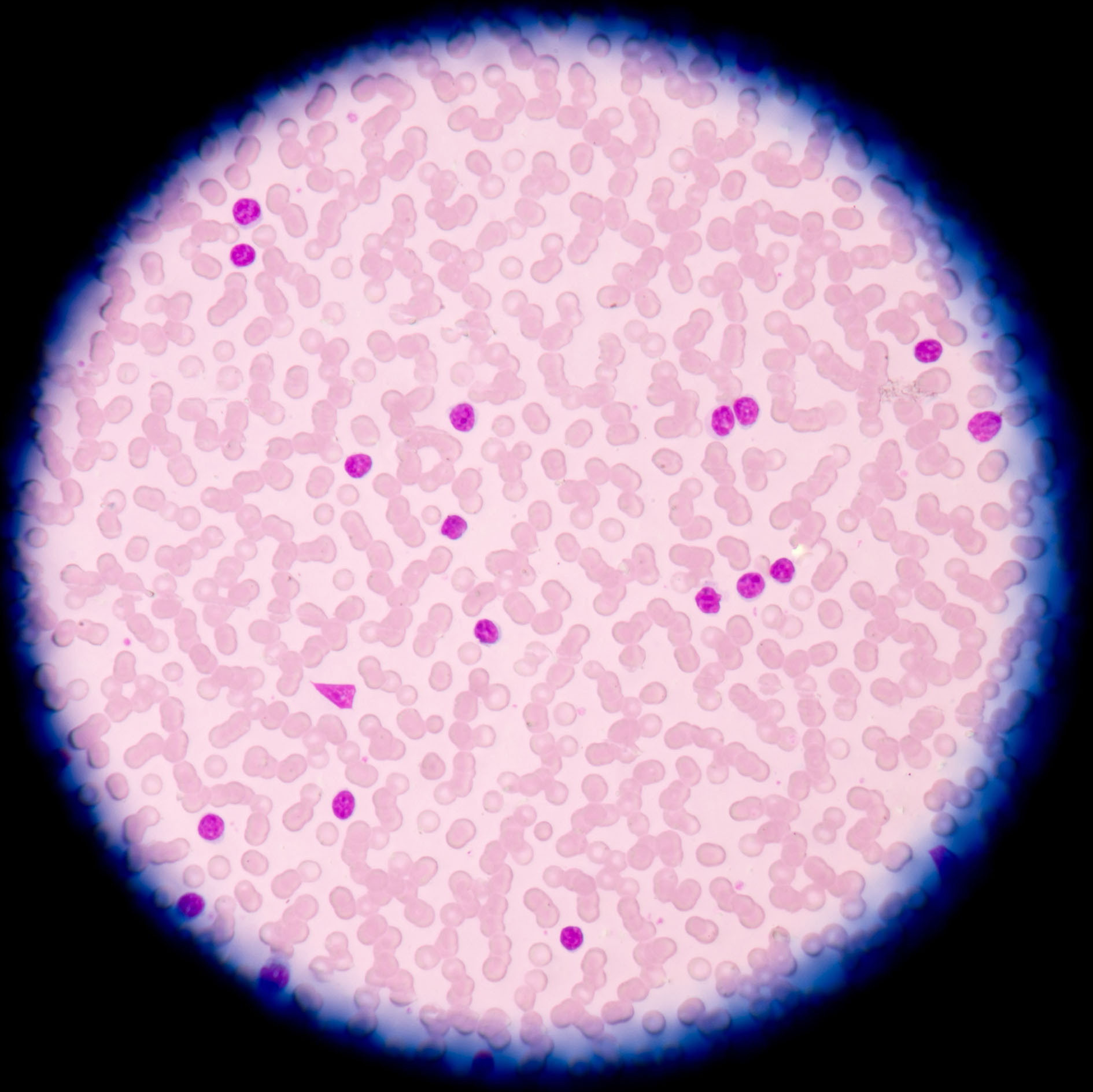
CLL is a type of cancer in which the bone marrow makes too many lymphocytes (a type of white blood cell). The disease, which is one of the most common to afflict adults, usually worsens gradually.
The pharma firm had hoped to push for accelerated approval for the treatment. It will now focus on its primary endpoint of progression free survival (PFS) to support full approval of ublituximab plus umbralisib (U2) combination.
The DSMB did not provide any further guidance but it will meet quarterly over the coming year and will continue to monitor the ongoing progress of the trial. TG did not reveal the specifics of the ORR.
Michael Weiss, executive chairman, president and CEO of TG Therapeutics said: “While we are disappointed that we were not able to report positive ORR today, we feel that making the decision to focus on PFS, the primary endpoint for the study, is an important step to getting everyone aligned on the endpoint of this study that matters most to the company and its long-term shareholders.
“From a timing standpoint, we could have a PFS read out in 2019, and we remain extremely optimistic about the prospects for a successful PFS result.”
Working as individuals, DSMB members provide their expertise and recommendations on drug trials and can terminate them if necessary.












