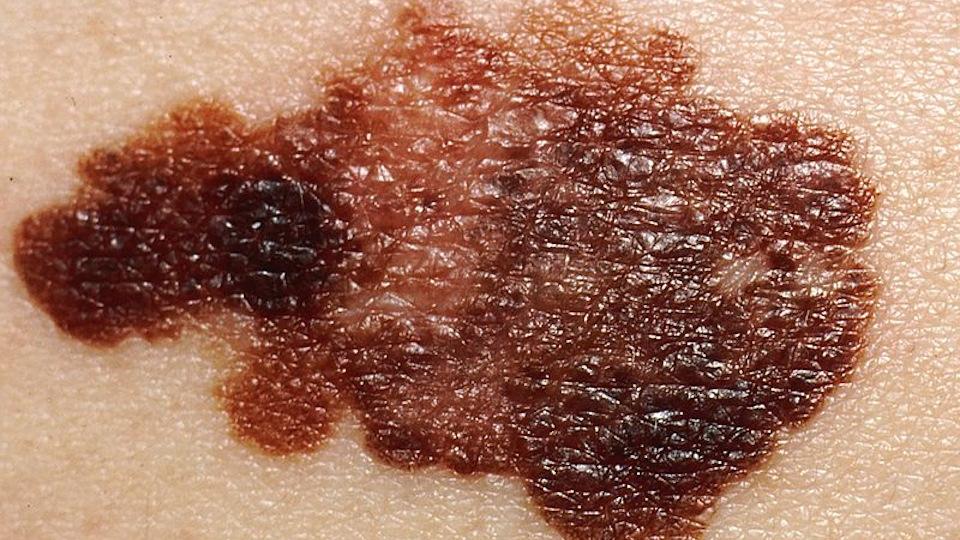
Scancell taps into NHS matchmaking service for cancer trial
UK biotech Scancell is using a new service launched by the NHS to pair patients with suitable clinical trials in a phase 2 trial of its melanoma cancer vaccine.
The SCOPE study of iSCIB1+, an off-the-shelf DNA plasmid vaccine that will be tested in combination with checkpoint inhibitors in advanced unresectable melanoma, will draw on the NHS Cancer Vaccine Launch Pad (CVLP) to drive recruitment.
Launched a year ago, the CVLP assesses people with cancer who are receiving treatment in the NHS in England to see if they might be eligible to join a cancer vaccine trial, but to date has focused mainly on mRNA vaccines – reflecting that it was set up in partnership with mRNA specialist BioNTech.
It has already begun helping thousands of patients to access trials of a personalised vaccine against bowel cancer, with more than 350 patients fast-tracked for consideration.
Scancell said that the inclusion of its DNA shot means that NHS hospitals across the country will be able to take part in the SCOPE study, and eligible patients from around the UK can now be referred to a participating NHS site. It also said it is the first UK company to participate in the programme.
SCOPE will test intradermal administration of iSCIB1+, which codes for various melanoma antigens from gp100 and TRP-2 proteins identified from T-cells of patients who achieved spontaneous recovery from melanoma skin cancers, in around 140 advanced melanoma patients.
It will be given in combination with immunotherapies which, while standard treatment, only works effectively in about half of patients with this form of skin cancer, leaving non-responders at risk of the disease progressing and spreading.
The hope is that the addition of the vaccine will raise that response rate, and clinical results with an earlier version of Scancell's shot given by intramuscular injection (SCIB1) have been promising.
Data due to be presented at the American Association for Cancer Research (AACR) meeting later this month in 25 patients will show an 84% disease control rate, 80% progression-free survival (PFS) with a 20% complete response rate, and a 72% objective response rate.
The CVLP partnership is being coordinated and run by the Southampton Clinical Trials Unit, with the first patients expected to be referred from May, according to Scancell.
"It's incredibly exciting that the NHS is expanding its world-leading programme so more patients with different types of cancer could benefit from the development of new vaccines that could stop their cancer coming back," said NHS National Cancer Director Professor Peter Johnson.
"We want to ensure as many eligible NHS patients as possible have access to these vital trials," he added.