NICE backs funding for Pfizer, Novartis breast cancer drugs
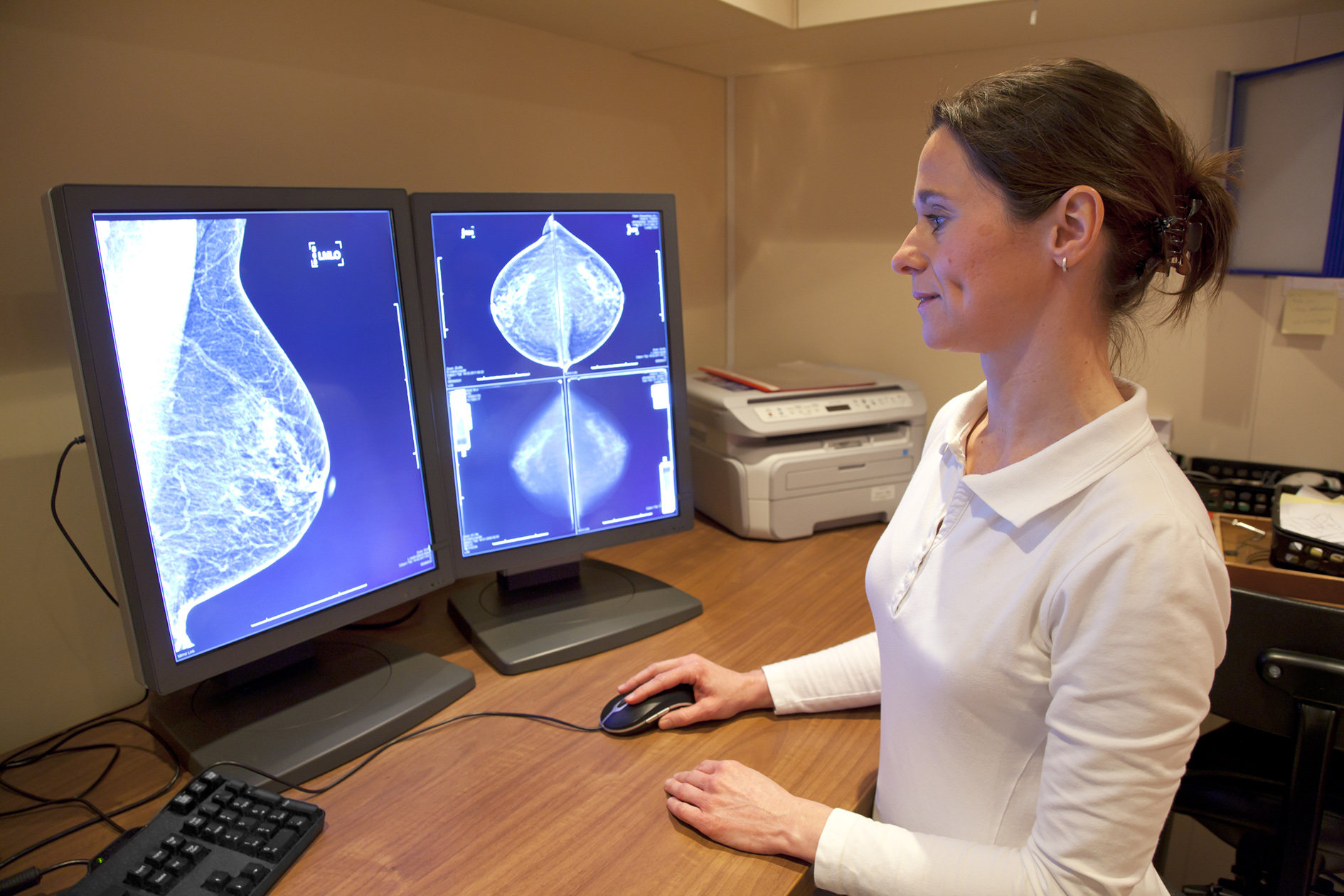
Women with an incurable form of breast cancer in England will get access to cutting-edge drugs after cost-effectiveness body NICE came to an agreement with manufacturers Pfizer and Novartis.
In final draft guidance, Pfizer’s Ibrance (palbociclib) and Novartis’ Kisqali (ribociclib) have both got the green light for National Health Service funding after the manufacturers agreed to cut their prices - although the confidential deals mean the size of these discounts remains unknown.
NICE also showed some flexibility in the way it deals with therapies that are added on to existing treatments. The news adds to a recent trend for more deals being struck between pharma, NICE and the Department of Health in the field of oncology.
NICE recommended both drugs, added to an aromatase inhibitor for hormone receptor-positive, human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer, as a first-line endocrine therapy in adults.
In terms of competition between the companies, the decision means Novartis has been successful in catching up with Ibrance in terms of market access in England and Wales, even though Pfizer's drug is already a global blockbuster and expected to earn $3.5bn this year worldwide.
Following behind is Lilly's Verzenio, (abemaciclib), which is not yet approved in Europe. The drug has only began the NICE process last month, meaning it will have some catching up to do on its rivals.
The drugs are more effective at halting progress advanced hormone-receptor positive, HER2 negative breast cancer than previous treatments, and can be used without chemotherapy and its unpleasant side-effects.
Pfizer had been at loggerheads with NICE since an initial rejection for Ibrance in February and took the unusual step of providing the drug free to patients in England until it could come to an agreement over funding.
Patients in Scotland will continue to get the drug without any charge to the NHS until 31 December, or the Scottish Medicines Consortium issues guidance, whichever is sooner.
Under NICE’s calculations the cost of the aromatase inhibitor is added to the cost of the add-on therapy, giving it far less flexibility.
However NICE said in its document that its independent appraisal committee was able to take a more “realistic” approach to treatment costs.
Both drugs inhibit cyclin-dependent kinases 4 and 6 and are priced at £2,950 for a 21-day supply. Pfizer and Novartis have both agreed to a commercially confidential discount with the Department of Health.
Nevertheless, Ibrance could gain the upper hand in England as elsewhere, as it carries fewer safety warnings than its rivals. Ibrance and Kisquali both raise the risk of neutropenia, but Kisquali also comes with heart rhythm risk and liver toxicity, while severe diarrhoea has been seen in patients taking Verzenio.
But a Pfizer spokesperson said there is more work to do to bring NICE up to speed with the latest advances in cancer drugs and improve patients’ access to the latest treatments.
The spokesperson told pharmaphorum in a statement: “Moving forward, we all need to continue working together to find ways of streamlining the process further. Cancer medicines are becoming more advanced and complex and if the UK is to achieve its ambition of world class cancer outcomes, we need to ensure our system is able to keep pace with medical innovation."
Novartis was able to make up ground on its rival because Kisqali is the first cancer drug approved using NICE’s faster appraisal system.
This sees assessments begin before Europe’s CHMP scientific committee has passed an opinion. The system aims to get a recommendation within 90 days of marketing authorisation – in this case it has taken 84 days.
Interim funding will now be made available until final guidance is published around the end of the year.













