NICE backs monthly eczema drug from Almirall
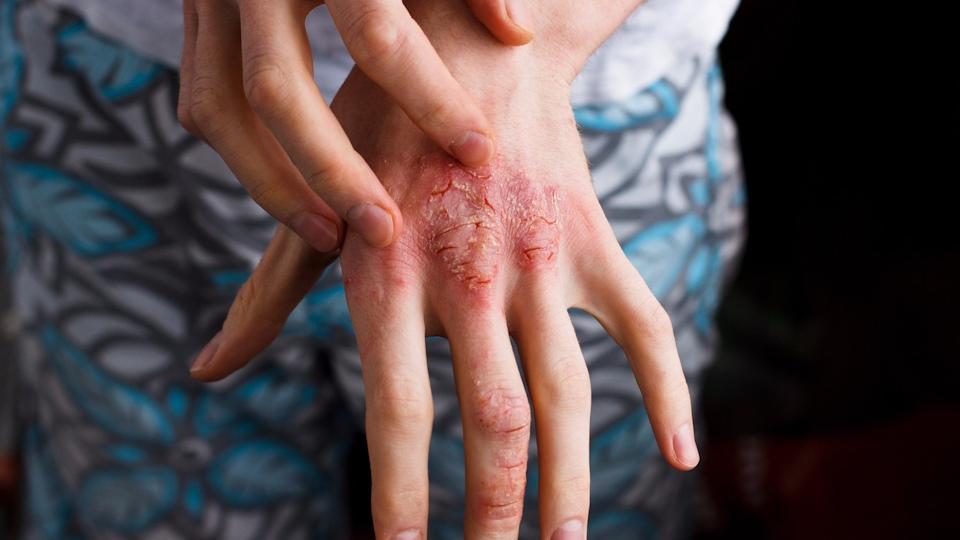
NHS England has been cleared to allow prescribing of Almirall’s Ebglyss for some people with atopic dermatitis, giving them the option of a therapy that only needs to be injected once a month.
IL-13 inhibitor Ebglyss (lebrikizumab) has been recommended (PDF) by cost-effectiveness agency NICE for people aged 12 and over with moderate-to-severe atopic dermatitis that has not responded to at least one system immunosuppressant treatment, or in cases where these are not suitable. It was approved for that indication by the UK drugs regulator last December.
The drug will enter the UK market as an alternative to Sanofi and Regeneron’s widely-used IL-4 and IL-13 inhibitor Dupixent (dupilumab), which was backed for atopic dermatitis in 2018 and requires dosing every two weeks, and other therapies. Ebglyss is given twice a week in the first month, then once every four weeks thereafter for those who respond to the drug.
The British Association of Dermatologists (BAD), which submitted comments to NICE’s appraisal committee in support of the drug, has said it will be an important additional alternative to previously available biologics like Dupixent and Leo Pharma’s IL-13 inhibitor Adtralza (tralokinumab), as well as various JAK inhibitors used to treat moderate to severe atopic dermatitis.
In trials, Ebglyss showed good and durable efficacy, with 80% of patients who responded to treatment at week 16 maintaining skin clearance and itch relief through one year of maintenance treatment.
Andrew Proctor, chief executive of the National Eczema Society, said that, while most people have heard of atopic dermatitis, they often “don’t realise how it can dominate the lives of patients and their families.”
Along with itchy, sore, cracked, bleeding skin, it can have a wide-ranging impact on their daily lives, “from what to wear, activities they can and cannot participate in, to managing time-consuming, messy skincare regimens,” he continued. “It’s important we have a range of treatment options, so patients have the chance to access a treatment that works well for them.”
Almirall – which has said that Ebglyss is its most important new product launch in years – also points to a good safety profile for the drug, including a low rate of conjunctivitis, which is a recognised side effect with Dupixent.
The drug has also been approved in the EU, although it was turned down by the FDA in the US, where rights are held by Eli Lilly, due to compliance issues at a third-party manufacturer.
Analysts at Evaluate have previously predicted that Ebglyss could become a $1.5 billion-a-year product, which would be transformative for Almirall, given that it made total revenues of a little under $1 billion last year.












