GSK drug raises hopes for patients with PBC itch
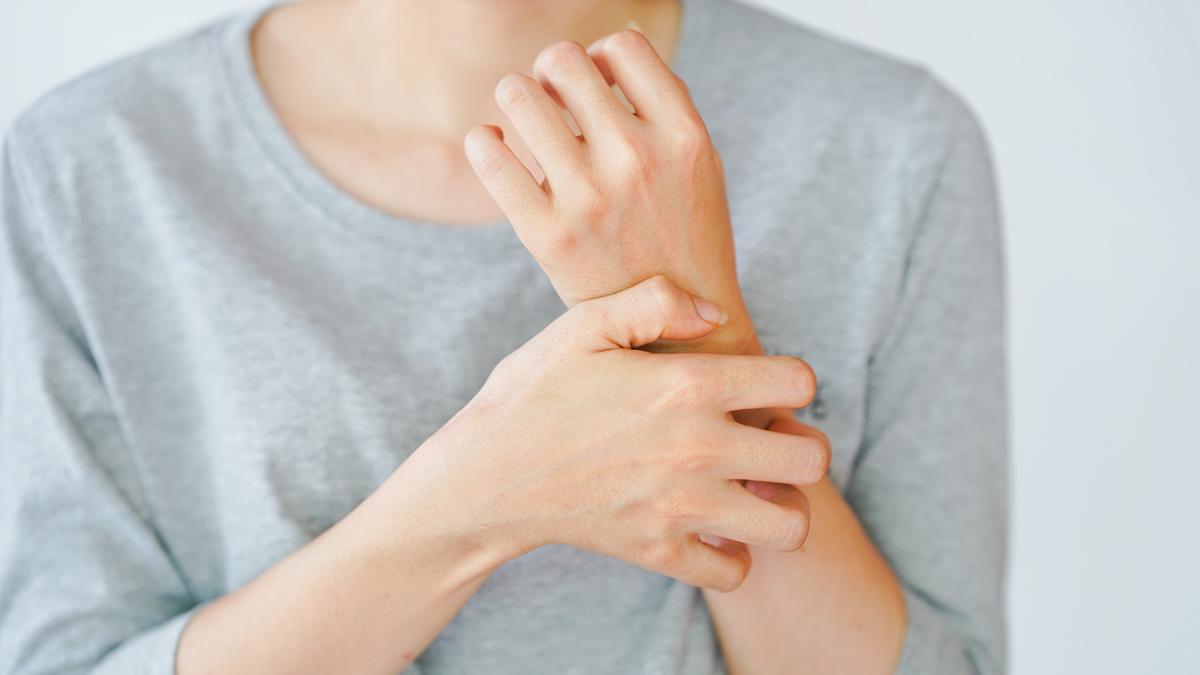
GSK has reported promising results with a drug to treat the severe itching that plagues patients with rare cholestatic liver disease primary biliary cholangitis (PBC).
Interim results from the ongoing GLISTEN trial of linerixibat, GSK's targeted inhibitor of the ileal bile acid transporter (IBAT), show that the drug achieved a statistically significant improvement in itch over 24 weeks compared to placebo in adult patients with cholestatic pruritus (relentless itch).
The study is recruiting patients with documented PBC and moderate to severe itching, who can either be treatment-naïve or already receiving standard itch therapies like colestyramine and antihistamines.
GSK said that linerixibat has a chance of becoming the first 'global' therapy for itch associated with PBC – in other words, it may be able to be used as a first- or later-line therapy.
There was little progress in the treatment of PBC for years until Ipsen's PPAR agonist Iqirvo (elafibranor) was approved in the US, EU, and UK this year for adults with PBC who have an inadequate response to ursodeoxycholic acid (UDCA), the standard first-line therapy for the disease.
Iqirvo was joined soon after in the US by Gilead Sciences' Livdelzi (seladelpar), another PPAR agonist with the same second-line indication, with both drugs competing with Intercept Pharma's Ocaliva (obeticholic acid), which first reached the market in 2016.
In trials, the PPAR agonists were effective in reducing progression of PBC, but did not achieve a statistically significant reduction in itch compared to placebo. Meanwhile, UDCA is also known to be fairly ineffective in treating itch - which is considered to be one of the most debilitating symptoms of PBC, with a huge impact on patients' quality of life - while itching is a recognised side effect of Ocaliva.
That presents a major opportunity for linerixibat if its effect on PBC itching is backed up in further data readouts.
PBC is a chronic, autoimmune disease in which bile ducts in the liver are gradually destroyed, leading to cirrhosis and sometimes cancer. According to GSK, the number of patients with the disease is expected to reach 510,000 worldwide by the end of the decade.
Of these, around 240,000 will experience relentless itch requiring treatment, according to the company.
"The itch associated with PBC for many patients is unrelenting and often severe, but is a symptom that is frequently overlooked or dismissed," said Carol Roberts, president of patient advocacy group The PBCers Organization.
"It has a significant impact on quality of life and mental health for people with PBC," she added. "The potential of a treatment option that addresses a root cause of itch answers a previously unmet need for people with PBC."
Ipsen also has an IBAT drug on the market – Bylvay/Kayfanda (odevixibat) – although, that is currently approved to treat itching associated with two other rare liver diseases, Alagille syndrome (ALGS) and progressive familial intrahepatic cholestasis (PFIC).
Ipsen is currently not running studies of odevixibat in PBC, but has a phase 3 programme on the go in biliary atresia.












