Grail plans FDA filing for cancer blood test after new trial
Grail is preparing to file for FDA approval of its Galleri test for detecting multiple cancers from a single blood test after seeing positive results in a second phase 3 trial.
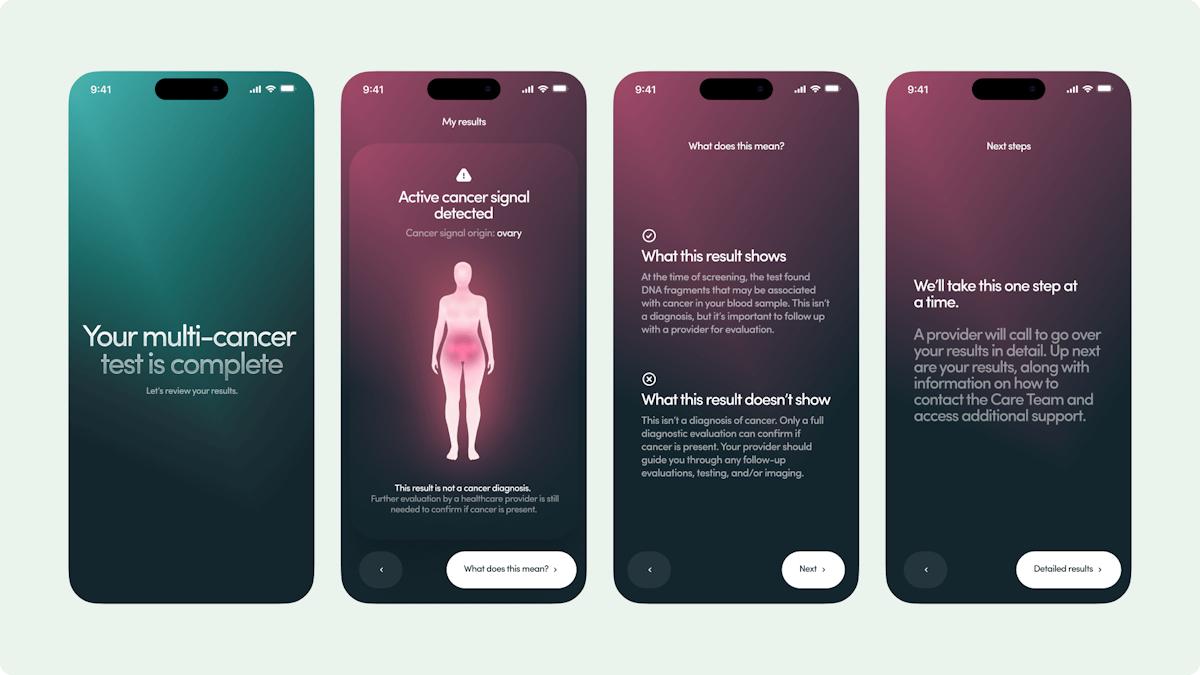
Galleri is a multi-cancer early detection (MCED) or 'liquid biopsy' test that Grail hopes will be able to spot signs of up to 50 different types of cancer in the early stages, when patients are more likely to respond to treatment. It works by detecting chemical changes in fragments of cell-free DNA (cfDNA) that leak from tumours into the bloodstream.
The new PATHFINDER 2 trial data comes from almost 26,000 people out of a total study cohort of around 36,000, all aged over 50 with no clinical suspicion of having cancer, with a Galleri test added to standard-of-care screening for a single type of cancer, such as colonoscopies or medical imaging.
The earlier PATHFINDER study in around 6,600 people found that adding the Galleri test to standard screening more than doubled the number of cancers detected, but Grail says its new study has eclipsed that result.
In the first study, which was published in The Lancet in 2023, Galleri demonstrated a positive predictive value (PPV) – in other words, the likelihood that a positive Galleri test was confirmed to be cancer – of 43%.
It also showed a specificity for the test of more than 99%, meaning it could accurately detect when patients did not have cancer, and 88% accuracy in cancer signal origin (CSO), i.e., correctly identifying where in the body a malignancy started.
The PATHFINDER 2 data isn't ready to be released yet, but Grail said in a statement that it showed a "substantially higher" PPV than was observed in PATHFINDER, while CSO accuracy and specificity were consistent with the earlier study.
The company said it will now share the results with the FDA as part of the Galleri premarket approval application (PMA), along with preliminary data from a screening study in 140,000 volunteers aged 50 to 77, conducted with the UK NHS, that is being run in 150 locations across England, with final results expected next year.
Grail said it will also submit data on bridging analyses that are comparing the original version of the MCED test used in the NHS-Galleri and PATHFINDER 2 studies to an updated version that it intends to submit for approval. A modular submission under an FDA breakthrough device designation is already underway and will be completed with the new data in the first half of 2026.
California-based Grail was formerly part of DNA sequencing giant Illumina, which bought the company for $7.1 billion in 2020, but was divested last year after the takeover was challenged by antitrust regulators.