FDA OKs wider use of Almirall’s actinic keratosis drug
With actinic keratosis (AK) diagnoses on the rise, there is some good news for patients, with the FDA approval of expanded use of Almirall’s topical therapy Klisyri.
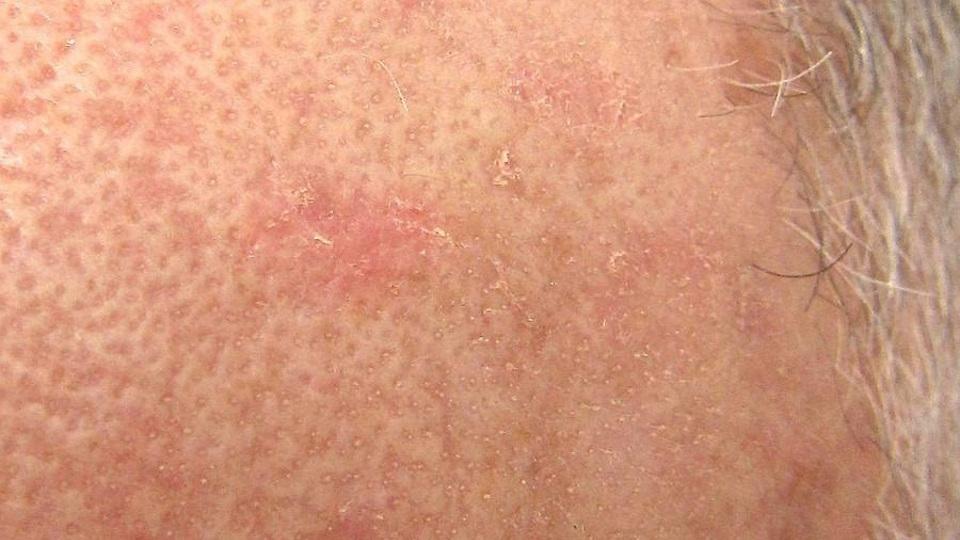
First approved in 2020, first-in-class microtubule inhibitor Klisyri (tirbanibulin 1%) reached the market in early 2021, joining a short list of therapies in the US that are available for AK, which is a major public health concern as it can be a precursor to skin cancer.
AK – also known as solar keratosis – is the second most common diagnosis made by dermatologists in the US with a reported prevalence of between 11% and 25%. It tends to take the form of rough, scaly patches of skin that appear on areas of the body exposed to ultraviolet light, such as the scalp in the case of balding men, the face, forearms, neck, or back of the hands.
Early diagnosis and treatment are imperative, as around 60% of squamous cell carcinoma (SCC) cases – one of the most common forms of skin cancer – start out as an AK lesion.
When it was first approved, Klisyri was labelled for use on areas of the face or scalp of up to 25 cm2, but it is now an option for patients with more widespread lesions of up to 100 cm2.
It is a significant approval for Almirall as it brings Klisyri into line with some other commonly used therapies for AK like PharmaDerm/Fougera’s Solaraze (diclofenac 3%) Gel and Graceway Pharma’s Zyclara (imiquimod 2.5%), which can also be used for lesions above 25 cm2. The company also says that the cream has the shortest application period (five days) for any topical treatment for AK.
“With patients experiencing AK over larger surface areas, dermatologists are looking for ways to treat the entire affected area to help prevent further lesion progression,” commented Karl Ziegelbauer, Almirall’s chief scientific officer.
Klisyri will now be available in a 350 mg package size, which can be used as a five-day topical treatment for AK, adding to the current 250 mg formulation.
The new approval is based on a phase 3 trial in patients with more extensive lesions that showed consistent results with the original pivotal trials conducted on an area of 25 cm2 and similar tolerability.
Of course, prevention of AK is much better than treatment, so the advice is not to go into the sun without sunscreen or other protection. However, a poll carried out by Almirall revealed that around a third (34%) of people who practised outdoor pursuits did not use sunscreen often, and more than half (52%) of them got sunburnt at least once a year.
Image by Future FamDoc via Wikimedia.












