FDA clears RHYTHM AI's new algorithm for atrial fibrillation
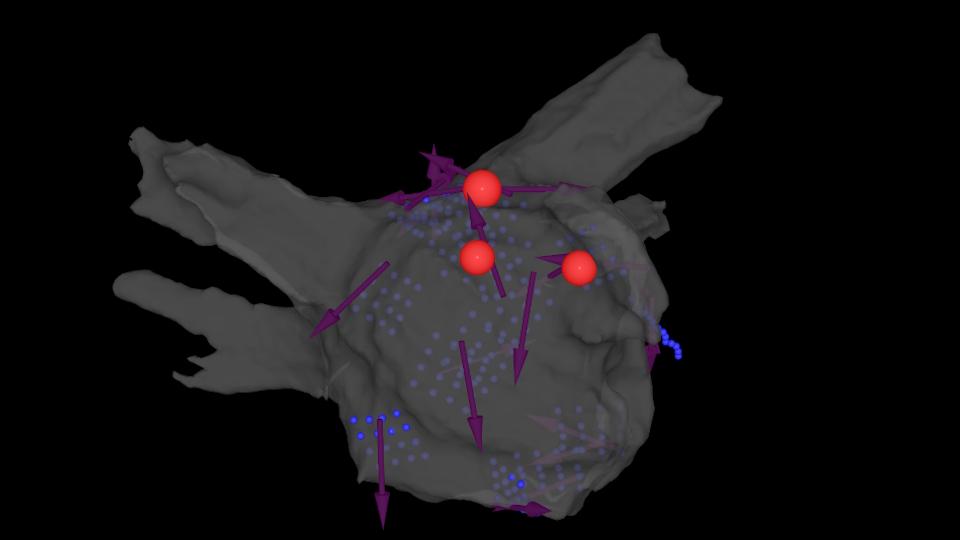
UK digital health company RHYTHM AI has claimed FDA approval for a new version of its algorithm for mapping areas of the heart that are affected by atrial fibrillation (AFib) that will be rolled out commercially.
The company has been refining its algorithm over several years, hoping to come up with a version that will support the treatment of patients with AFib who no longer respond to medication.
Antiarrhythmic drugs are standard therapy for AFib, but often only work for around 18 months, after which time a minimally invasive procedure called catheter ablation is necessary to isolate the location of the electrical abnormality that causes the heart rhythm disorder. Using heat or freezing, the ablation is used to create scar tissue that blocks the aberrant electrical signals in the heart.
Catheter ablation is the most common and most effective treatment for AFib but, even in experienced hands, the first-time success rate is usually only around 50%, according to London-based RHYTHM AI.
The STAR Apollo algorithm has now been cleared for use alongside Abbott's EnSite X EP cardiac mapping system, a widely-used technology that provides a 360-degree image of the heart and is used to direct catheter ablation procedures for abnormal heart rhythms.
In real-time, Apollo Star can be used to reveal repeating patterns within the arrhythmia and identify regions of the heart that are potential drivers of AFib, allowing the creation of a customised treatment plan for each patient.
AFib is the most common heart rhythm disorder, affecting more than 40 million people worldwide, and estimates suggest that around one in three people in the US and Europe will develop the condition during their lifetime. It is a major cause of cardiovascular illness and raises the risk of complications, including stroke.
Alongside the FDA's 510(k) clearance, RHYTHM AI has also reported the first uses of the new version of the technology to treat AFib patients – at Baptist Health in Jacksonville, Florida – with successful results.
The physician who carried out the procedures, Dr Matthew McKillop, previously participated in an observational study using an earlier version of STAR Apollo and is involved with a clinical trial of the system in patients who continue to have AF despite an earlier ablation procedure.
"STAR Apollo is allowing us to build tailored and targeted treatment strategies for this large patient group," commented McKillop. "It gives me unique insight into an individual's [AFib]; I'm excited to use this new technology for my patients."












