From representation to empowerment: Putting patients at the centre of medicine reimbursement

Experts from Vynamic discuss their vision for a future where patients are at the heart of medicine reimbursement in Europe – and provide actionable steps for achieving this.
This article appears in full in our digital magazine Deep Dive: Market Access 2021. Read below for a sneak-peak:
The reimbursement landscape in Europe is complex. There are different models, medicines are evaluated in different ways and the timelines for approval vary widely. Most crucially, the level of patient involvement is not where it needs to be. This simply has to change.
Patients provide the human face for evidence – identifying outcomes that are important to them, addressing gaps in the clinical evidence base, helping verify or refute assumptions in economic models and informing the determination of added value.
Vynamic’s vision for the future is one that places the patient at the heart of the reimbursement process across the full value chain – but to achieve that vision, all stakeholders need to advocate for major changes across the entire sector, and there are many implications to consider before embarking on such a journey.
The degree to which patients and patient organisations (POs) can be involved in the HTA review process varies across Europe, and there are as many situations as there are countries.
Some countries like France or Sweden have a formalised process where patients and POs can vote within HTA committees. In contrast, other European countries like Austria, Italy or Portugal do not usually share HTA information nor consult patient representatives. Following the appraisal of a medicine, in some instances patients and POs have the right to appeal or provide input at re-evaluations.
Progress is being made and there is a strong foundation to build upon. However, much more needs to, and can, be done.
Underlying our vision is the concept of augmenting patient empowerment through the lens of ‘No decision about me, without me’. We want to see a future where patients and POs are at the centre of medicine development and reimbursement decisions, as they represent real-world patient perspectives and needs. This will help close the gap between “hypothesis” and “reality” when it comes to improving disease outcomes and enhancing health and lives.
To achieve this, stakeholders will need to rethink every aspect of medicine development and HTA.
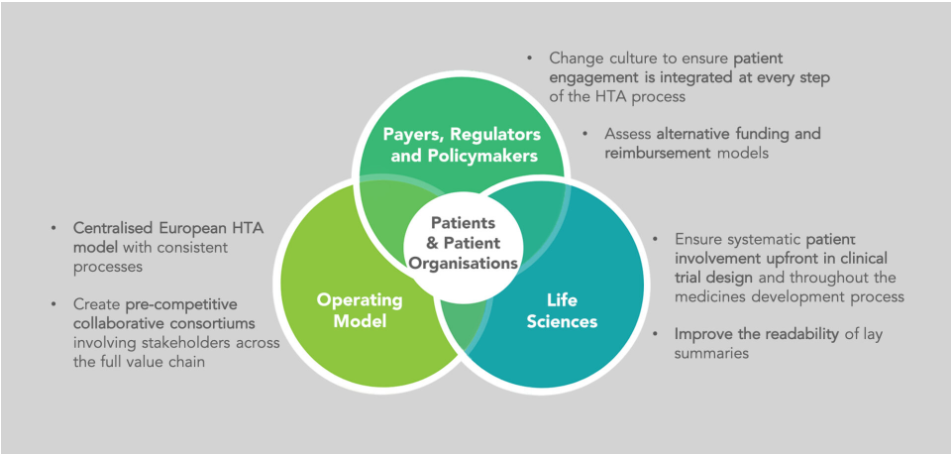
All stakeholders should look to establish and adopt a centralised European HTA operating model, building on the cross-border HTA collaboration led by EUnetHTA. This will help drive consistent processes, effective assessments and enable early access to high-value medicines. It will also be important to create pre-competitive collaborative consortiums involving stakeholders across the full value chain, so that they can share lessons learnt and establish best practices.
Meanwhile, payers, regulators and policymakers need to advocate for changes in culture that ensure patient engagement is integrated at every step of the HTA process – as well as assessing alternative funding and reimbursement models to continue to improve patient access to licensed treatments.
Finally, the life sciences industry needs to be ensuring systematic patient involvement upfront in market and disease assessments, clinical trial design and throughout the medicines development process. To facilitate this, readability of lay summaries needs improvement to maximise the value of information exchange and educate POs. This will empower them to provide greater input and influence over HTA decisions.
Most critically, patients and POs should also be looking to engage with these stakeholders as often as they can to advocate and lobby for these changes.
There is a long road ahead for implementing such wide-reaching changes, but the result will be well worth the journey – with better access to treatment, improved ways of working and, ultimately, better patient outcomes.
• Read the full article in pharmaphorum's Deep Dive digital magazine













