ETH develops AI algorithm for drug discovery based on 3D protein surface

Chemists at ETH Zurich have developed a new computer process that enables the generation of active pharmaceutical ingredients at speed, based on a protein’s three-dimensional surface.
The new generative AI (GenAI) develops molecules from scratch in a way that precisely matches the protein they are intended to interact with. Thought to offer a potential to “revolutionise drug research”, the new computer process ensures that the molecules can also be chemically synthesised.
“It’s a real breakthrough for drug discovery,” said Professor Gisbert Schneider, of ETH Zurich’s Department of Chemistry and Applied Biosciences.
Together with his former doctoral student Kenneth Atz, Prof Schneider developed an algorithm that uses AI to design new active pharmaceutical ingredients. For any protein with a known three-dimensional shape, the algorithm generates the blueprints for potential drug molecules that increase or inhibit the activity of the protein. Chemists can then synthesise and test these molecules in the laboratory.
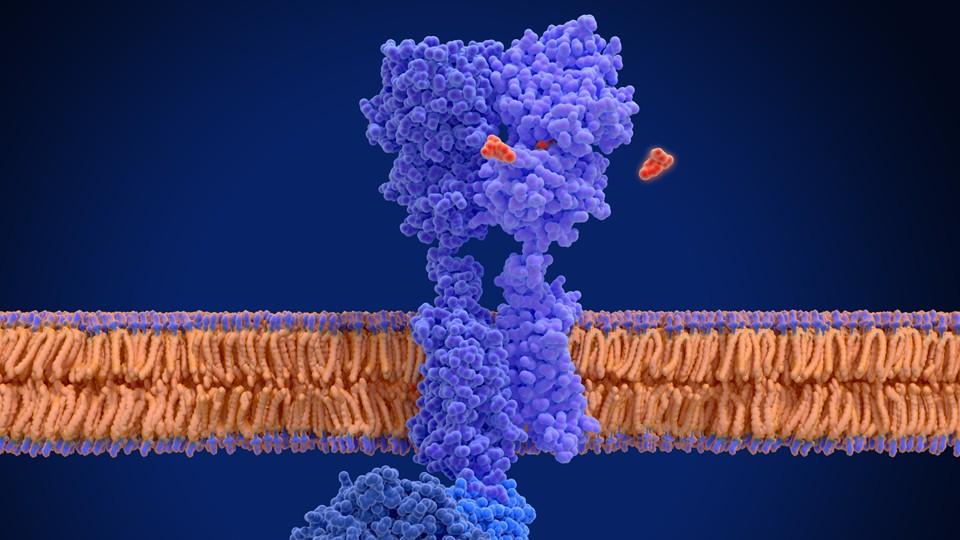
Based on a protein’s three-dimensional surface structure, the algorithm designs molecules that bind specifically to the protein according to the lock-and-key principle, so they can interact with it.
To create the algorithm, the scientists trained an AI model with information from hundreds of thousands of known interactions between chemical molecules and the corresponding three-dimensional protein structures.
The new method builds on decades-long effort to elucidate the three-dimensional structure of proteins and use computers to search for suitable potential drug molecules. Until now, this has been done manually, and yielded molecules that were very difficult or impossible to synthesise. GenAI accelerates and ameliorates the process.
“This means that, when designing a drug molecule, we can be sure that it has as few side effects as possible,” commented Atz.
Together with researchers from Roche and other cooperation partners, the ETH team tested the new process, searching for molecules that interact with proteins in the PPAR class – proteins that regulate sugar and fatty acid metabolism in the body. Several diabetes drugs used today increase the activity of PPARs, which causes the cells to absorb more sugar from the blood and the blood sugar level to fall.
The AI designed new molecules that also increase the activity of PPARs, like drugs currently available, but without a lengthy discovery process. After the ETH researchers had produced these molecules in the lab, colleagues at Roche subjected them to a variety of tests, demonstrating the new substances are stable and non-toxic - right from the start.
The algorithm is already being used for similar studies at ETH Zurich and in industry. One of these is a project with the Children’s Hospital Zurich for the treatment of medulloblastomas, the most common malignant brain tumours in children. Moreover, the researchers have published the algorithm and its software so that researchers worldwide can now use them for their own projects.
“Our work has made the world of proteins accessible for generative AI in drug research,” said Prof Schneider.
Working in collaboration with EPFL, ETH has also launched the Swiss AI Initiative. This aims to position Switzerland as “a leading global location in which to develop and use transparent and trustworthy AI”.
Meanwhile, another company venturing forth with AI processes is Moderna, which has teamed up with OpenAI, the artificial intelligence company behind ChatGPT, to further integrate GenAI across its mRNA drug development and manufacturing operations.
Image by Google DeepMind.