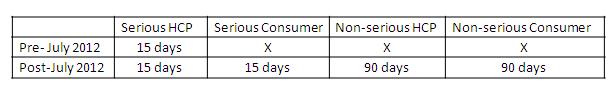Are you ready for the new EU pharmacovigilance legislation changes? Part 1

Graeme Ladds
PharSafer
Today we have an article from Graeme Ladds about this year’s EU pharmacovigilance legislation changes and the affects this will have on pharmaceutical companies and manufacturers. This article explores previous European and National laws, looks at expedited reporting by healthcare professionals and the Periodic Safety Update Report.
There have been progressive changes in legislation since the emergence of Vol IXa in January 2007 (first appearance of many) for pharmacovigilance activities for licence holders in Europe. This is to keep the legislation moving forward in terms of safety reporting and the expectation of the analyses of such data to ensure that the pharmaceutical industry and the regulators have helped keep the pharmacovigilance (PV) activities developing while in the background new philosophies for additional PV activities were being developed.
The launch of legislation 1235 / 2010 and 2010 / 84, including the sixteen sets of guidance notes, has meant that from July 2012 the next evolutionary stage of PV for companies with licences in the EU begins. Rather than evolving from Vol IXa, this set of legislation has radical departures from conventional PV and pushes European PV into new areas as this two-part article will discuss.
The Legislation
As mentioned, this has required new laws to have been passed (1235 / 2010 &, 2010 / 84) and the guidance notes (sixteen in total) which are equally applicable to the law, basically describing what the expectation is of the licence holder in terms of pharmacovigilance activities.
The legislation has passed from a European level to a National level, being transposed into National law throughout Europe by July 2012. It is anticipated from the EU legislation that the transposition into National law will not be accompanied by additional National requirements, but with a few months to go this is still an unknown phenomenon.
Expedited reporting
The premise for most expedited reporting in the EU to date has involved qualification of the report by a healthcare professional (doctor, pharmacist, dentist, nurse).
Therefore, pharma companies have always sought healthcare professional (HCP) verification of any reports submitted to them by patients or patients’ relatives etc.... There were a few countries that recently allowed consumer / patient reports to be submitted as expedited reports and these were Denmark, Germany and Hungary.
 ,
"The premise for most expedited reporting in the EU to date has involved qualification of the report by a healthcare professional..."
 ,
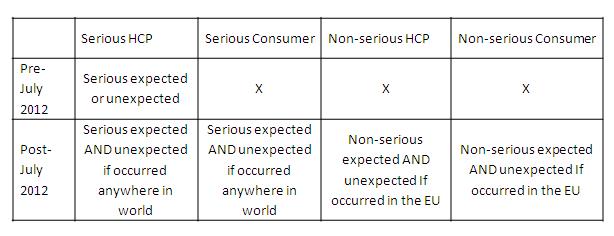
Equally, an expedited report also had to be classified as ‘serious’ in order to be expedited. This required the reporter to define the event(s) as serious or the Pharma Company to assess an undefined report as being serious. The seriousness criteria were based upon the ICH definitions taken for clinical trials from ICH E2A and for post-marketing from ICH E2D.
The timelines for reporting an expedited report were also encompassed in these ICH documents and for clinical trials this was seven calendar days for fatal / life-threatening events (and an eight day follow up) and all other unexpected serious reports in 15 calendar days.
For post-marketing cases, the reports have to be submitted electronically in the EU within 15 calendar days of being presented with a reporter, event(s), company suspect product, and patient details (four minimum criteria).
Under the new legislation there are marked extensions to these expedited reporting requirements as mentioned in the Table below:
The timelines for reporting (calendar days) for these events are as follows:
Equally, and according to National regulations, the type of serious reports to be expedited varied between serious expected and unexpected, whereas for the above rules the following is true:
Therefore, the number of reports that will be submitted to the Regulatory Agencies in the EU post July 2012 will dramatically increase, as will the amount of reporting from the pharma companies.
Generally, in a pharma company, the number of reports received can be split as 40% serious HCP, 20% serious consumer and 35% non-serious consumer and less than 5% non-serious HCP. Therefore, previously only 40% of what a pharma company received in terms of adverse event reports was submitted to the agency and so an increase of nearly 60% extra can be expected from the new legislation.
So, what is hoped to be gained from requiring such additional reports to be now supplied to the agencies?
There are a number of ways of utilising the extra data obtained from the additional reports being received. The most important is signal detection. With more reports being received, the Regulators can perform more quantitative signal detection activities which does involve (at least in the preliminary stages) using large numbers of data and looking for differences in the data such that types of reactions are more common in some products than others. This quantitative approach of identifying such anomalies means that having identified a possible anomaly, it is then important to look at the individual case reports to look for commonalities of each case, and observing possible patterns and similarities that would help confirm the positive causality.
It is the EMA’s intention to allow companies to see the results of the various quantitative signal results and indeed there is a guidance note in the EU already concerning the use of quantitative methods for signal detection.
The second reason is that if all non-serious cases are now submitted it will be easy for the Agency to determine if there has been a correct assessment of seriousness by the Company.
 ,
"Under current legislation the requirement to perform literature searching of scientific literature at least once per week falls upon the Marketing Authorisation Holder."
 ,
The third reason is that the data provided by HCPs will tend to provide diagnoses and medical terms and from patients these will be signs and symptoms. By combining the two types of reports in MedDRA queries (specifically Standard MedDRA Queries (SMQs) used to aid signal detection) then this also aids signal detection.
Literature searching
Under current legislation the requirement to perform literature searching of scientific literature at least once per week falls upon the Marketing Authorisation Holder (MAH).
Under the new proposed legislation, some of this literature searching for products by the EMA using an EMA-contracted-company will be performed by looking at individual case safety reports (ICSRs). These will be searched and entered into the EMA Eudravigilance database and copies provided to those MAHs affected.
However, currently, there is very little published detail on how this will operate and the literature searching proposed would not pick up some literature articles referring to data associated, for example by risk, where patients may be at increased risk of developing AEs and this may be a class effect. Effectively, this means that the MAH still has to perform literature searching as well.
The EMA has said that as a result of providing this literature searching service for the MAHs there will be a charge applied, but to date no scale of charges has been provided.
Also, there are many literature articles that from the title or the abstract do not provide any suggestion that an individual case may be found inside the article.
As a result, if the MAH now orders an article and there are identified cases within the article, no mention has been made as to whether this requires the MAH to report them as ICSRs.
The Periodic Safety Update Report (PSUR)
PSURs have been part of the legislation in Europe for nearly 20 years and were based upon the ICH guidance document ICH E2C and its subsequent update in 2003. The requirements for submissions of PSURs were located in the EU legislation in the old Vol IX as well as the various versions of Vol IXa which came out in 2007 and the last version was Sept 2008.
There have been a number of EU initiatives with regards to PSURs which has involved a unification of birth dates for generic products so that all generic products have their PSURs submitted at the same time.
There has been an approach to unify all labelling for generic products to reduce anomalies for essentially the same product. Such approaches are underway and will continue under the new legislation.
Additionally, under the new legislation there has been a streamlining proposal not to submit PSURs for certain types of products, which will include herbal and homeopathic medicines as well as established generic products.
 ,
"Additionally, under the new legislation there has been a streamlining proposal not to submit PSURs for certain types of products..."
 ,
Therefore, after July 2012, there will be three parallel activities underway for PSURs, unification of submission dates, unification of labelling and notification of the lack of requirement to submit PSURs for certain products. It is the responsibility of the MAH to monitor these activities as published on the EMA website.
For those PSURs still left for submission, there are a number of proposed changes in the guidance notes that will require careful review by the companies, because of the extent of the proposed changes to the format of the PSUR will also require a major re-think in the production, review and assessments made within the PSUR.
Much of the revisions to the PSUR format and timelines have emanated from another ICH document that has now been adopted in the EU, USA and Japan and this is ICHE2F, which covered the Developmental Safety Update Report (DSUR).
The DSUR document replaced the Annual Safety Report submitted as part of the Clinical Trial Directive (2001 / 20) and has been fully adopted in the three ICH Regions mentioned above. Unlike the Annual Safety Report or the Investigational New Drug (IND) annual report in the USA, this document places far more emphasis on the potential analyses of safety findings rather than the previous documents where presentation of all of the safety information was the key criterion.
The DSUR requires the sponsor to explain the processes and deliberations for potential safety signals and what, if any, new safety signals have meant to the overall benefit: risk profile. Again the emphasis of the benefit: risk calculation is to explain how this was calculated so that rather than provide an overall conclusion to the benefit: risk profile, the regulators can read the sponsors methodology of calculating the benefit: risk profile to determine if they agree. The length of time to write up such a report (DSUR) still remains at 60 calendar days.
The template for the DSUR has many sub-sections dedicated to looking at potential signals, the determination of the signals and the calculation of the benefit: risk. This placed the DSUR document at a distinctly different level from the PSUR template and certainly in the level of data interrogation compared to the PSUR which remained mainly a presentation of data document.
 ,
"As a result of the new requirements for data analysis deliberations, the regulators have recognised that this will take a longer time to prepare for MAHs..."
 ,
Therefore, the proposal was made at regulator level to re-visit the PSUR document so that it much more resembled the DSUR document. This process is currently on-going for the ICH E2C document, but in the meantime, the PSUR guidance document for the new EU legislation precedes any deliberations for the ICH revision and does adopt the DSUR template.
As a result of the new requirements for data analysis deliberations, the regulators have recognised that this will take a longer time to prepare for MAHs and so not only does the template need changing, but so do the times for being able to prepare and submit the PSURs after the data lock point.
• For PSURs less than one year in length, the new timelines to prepare the PSUR have been extended from 60 to 70 days.
• For PSURs over one year old then the timelines is even longer being extended from 60 to 90 days.
From these two perspectives alone it is clear the regulators know the new format will take much longer to complete.
The renewal PSUR will also change, in that previously, there was a renewal PSUR submitted after 4 years and 6 months, but now the submission has to be made in 4 years and 3 months, giving the Regulators 9 months to review, rather than 6 months previously.
Also, now there will only be one renewal for a product unless there are on-going serious safety concerns, which may result in a further renewal submission.
Therefore, the PSUR document and some of the content will be lifted from the conclusions from safety review meetings to show how and when potential signals were identified, tracked, implemented or rejected. This will place a greater emphasis on the safety review groups to keep comprehensive notes to explain, for example, what was discovered, why it was accepted as a signal and how it was put into the labelling.
Read part two of this article here, to find out about quality management, the Pharmacovigilance Master File and what’s next for legislation.
 ,
 ,
About the author:
Graeme Ladds is the CEO of PharSafer® and has over 20 years experience in the pharma industry. His previous position in the industry was as Head of Global Drug Safety for a multi-national pharma company and as an EU Qualified Person for Pharmacovigilance (EU QP PV).
Graeme has worked as both a Pharmacovigilance and Medical Information Manager previously, and over the many years of working in the field of safety and medical services has written a modular book on multi-national pharmacovigilance (2006, 2010), written many articles on pharmacovigilance in peer reviewed journals, currently serves as editor on a multi-national pharmacovigilance journal, is a regular speaker at conferences, and active member of many international associations which include the DIA (and active member of SIAC), TOPRA, and ISOP.
PiR Resourcing is a brand synonymous with providing innovative senior resourcing solutions to international life science organisations. As a result of our exclusive focus on the sector, we have an understanding of many of the issues faced by pharmaceutical, biotechnology, diagnostic and medical device companies in the 21st century. PiR Resourcing offer a range of resourcing services, core to these are senior permanent and interim staff.
• Permanent Resourcing Solutions
• Interim Management Services
• Talent Identification
• Board Evaluation &, HR services
• Non Executive Directors
PiR Resourcing’s expertise is particularly evident across functions in high demand. These include Medical, Regulatory, Programme Management, Supply Chain, Market Access, Health Economics &, Outcomes Research (HEOR), Pricing &, Reimbursement and senior level Commercial roles.
Email: resourcing@pir-resourcing.com Phone: +44 (0) 844 880 4340 Website: www.pir-resourcing.com
What do the proposed EU pharmacovigilance legislation changes mean to you?