Recent trends in ANDA litigation

As the numbers of Abbreviated New Drug Applications (ANDAs) rise in the US, what are the trends in litigation? Sheila Swaroop and Kaitlin Heinen examine the findings of a recent report.


Sheila Swaroop (left) and Kaitlin Heinen
The use of legal analytics to map general trends in litigation has become an increasingly popular tool. In a Hatch-Waxman/ANDA Litigation Report, published in April 2016 by legal analysts Lex Machina, data were analysed from approximately 2,200 cases filed in US district courts from January 2009 to December 2015. This article summarises the statutory framework for this litigation and highlights some of the report’s findings.1
Key takeaways
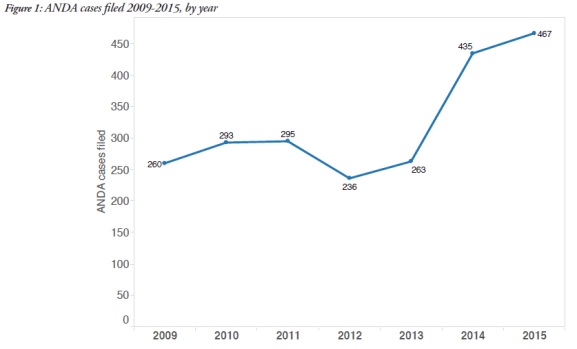
1. Filings of ANDA litigation on the rise
The report showed that an average of 269 cases were filed each year from 2009-2013, with a 68% increase, to 451 cases, in 2014-2015.
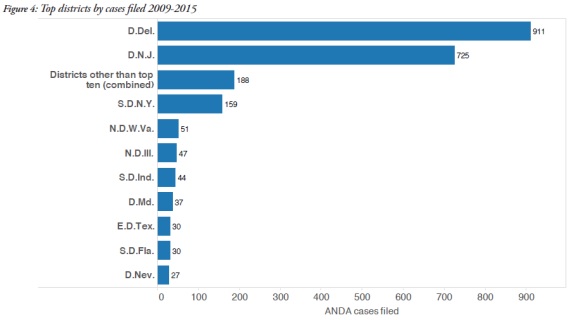
2. ANDA litigation primarily takes place in Delaware and New Jersey
The overwhelming majority of ANDA cases were filed in federal courts in Delaware and New Jersey. Delaware was the forum of choice for 911 cases, and 725 cases were filed in New Jersey. The figure below identifies the other districts in which ANDA cases were filed during the reported time period and demonstrates the predominance of Delaware and New Jersey.
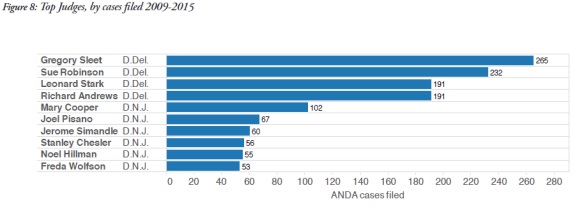
Many of the pharmaceutical companies which litigate ANDA cases are incorporated in Delaware or have headquarters in New Jersey, which probably explains the popularity of these two forums. In addition, the judges in these areas have become very familiar with ANDA litigation, as shown in the graphic below, and have presided over multiple ANDA cases.
3. ANDA cases involve repeat litigants
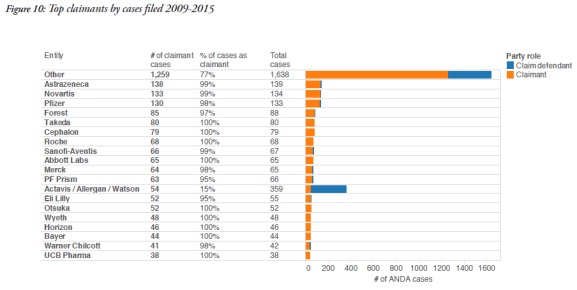
The report also identified the participants in these cases. On the patentee side, AstraZeneca participated in 138 cases as a claimant, Novartis in 133, and Pfizer in 130. Other frequent patentees included Takeda, Cephalon, Roche, and Abbott Labs.
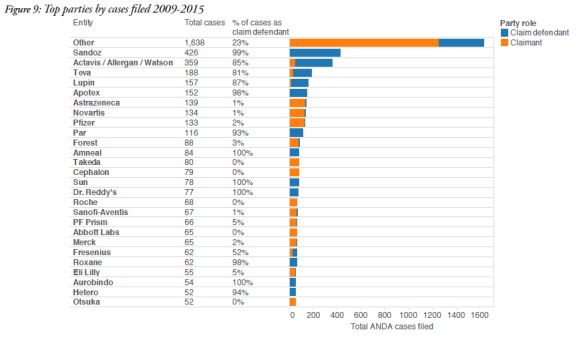
On the defence side, the report identified Sandoz as the most frequent participant, with 422 cases as a defendant. Actavis, which includes Allergan and Watson Laboratories, participated in 305 cases, and Teva participated in 152 cases. Other frequent defendants included Lupin and Apotex.
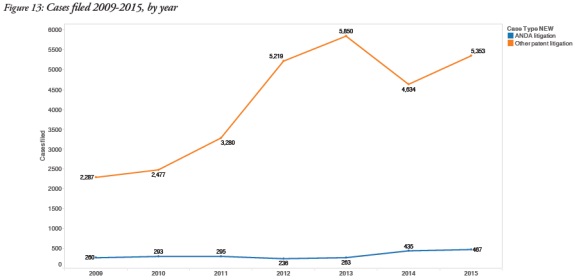
4. ANDA litigation trends distinct from other patent litigation
The report determined that ANDA litigation comprised 10% of all patent litigation in US district courts and maintained steadier filing rates than other types of patent litigation:
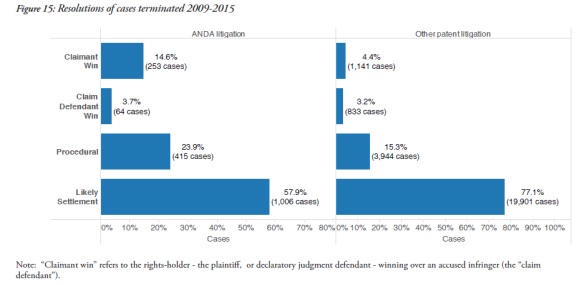
It found that ANDA cases were much less likely to end as a result of a settlement – 57.9% in ANDA cases, but 77.1% in other patent cases. The report also revealed that claimants had a win rate of 14.6% in ANDA cases, compared to a 4.4% win rate for other types of patent cases.
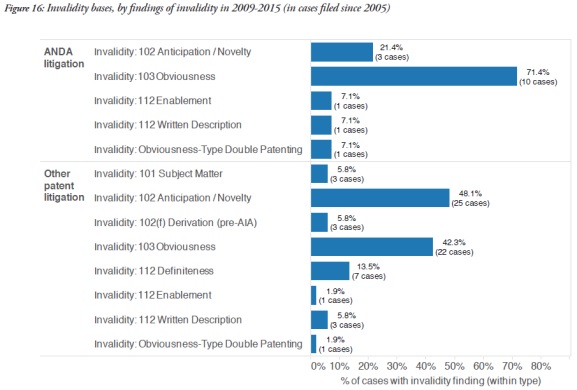
Obviousness, under 35 U.S.C. § 103, was the most frequent ground for finding invalidity of the patents involved in ANDA cases (71.4%), while other patent cases tended to rely on anticipation/novelty grounds, under § 102 (48.1%), when they were successful on the issue of invalidity.
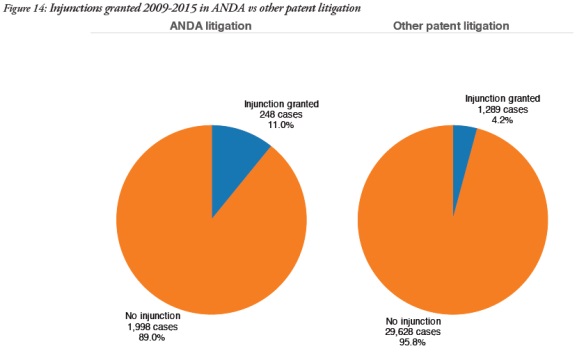
ANDA cases were significantly more likely to involve injunctions – 11% in ANDA cases but 4.2% in other patent cases. This is probably due to the fact that many ANDA settlements include the issuance of an injunction as one of the terms.
5. Timing of ANDA litigation consistent with the statutory 30-month stay
The median time to a claim construction, or ‘Markman’ hearing in ANDA cases, was 15 months, while the median time to trial was 27 months. These statistics correspond to the general framework for ANDA litigation, in which litigants aim to reach a substantive resolution of the patent claims within the 30-month stay of the ANDA that is put in place when the lawsuit is filed.
6. Increased use of concurrent patent office proceedings
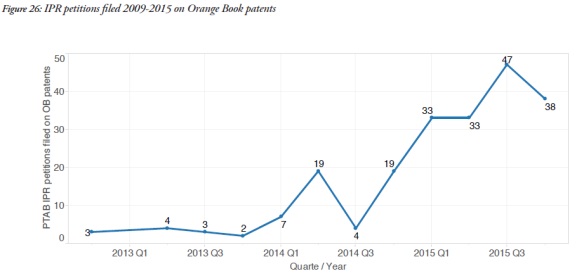
Another interesting trend reflected in the report was the increase in the frequency of invalidity challenges of Orange Book patents in concurrent Patent Office proceedings. Since 2012, ANDA applicants have had the ability to seek inter partes review (IPR) of patents at the Patent Trial and Appeal Board. The Report showed that the use of IPR proceedings to challenge these patents has been growing over time. That trend is expected to continue, in view of the difference in claim construction standards and lower burden of proof that govern IPR proceedings.
Overall, the report summarises some general trends for ANDA cases that are helpful when assessing forum selection, timing and strategies for Hatch-Waxman litigation.
Note:
- Findings from the Report are used with permission from Lex Machina.
About the authors:
Sheila Swaroop is a partner at Knobbe Martens Olson & Bear LLP in CA, US, where she focuses on litigation of intellectual property (IP) disputes. She has litigated Paragraph IV disputes under the Hatch-Waxman Act for pharma companies. Her experience includes pre-litigation counselling, strategic enforcement, and litigation of disputes through trial.
Kaitlin Heinen is a summer associate at Knobbe Martens Olson & Bear LLP and currently attends Boston University School of Law.
Read more on regulation:











