How immunotherapies are changing treatment of non-small cell lung cancer

Daniel Blake reviews the science supporting new therapies that are showing promise for non-small cell lung cancer and the challenges yet to be overcome.
This year is the year of immunotherapies, as demonstrated by the focus on these at the American Society of Clinical Oncology (ASCO) annual meeting. Of particular interest is how they affect the treatment of non-small cell lung cancer (NSCLC), it being the most commonly-diagnosed cancer globally1 across age groups and genders and most often diagnosed at an advanced stage2, often with limited prognosis.
As such, NSCLC has long been a focus of significant clinical development and is a poster child in the arena of personalised medicine; treatment varies significantly depending on the patient's tumour biology and molecular status (i.e. epidermal growth factor receptor [EGFR] mutation and Anaplastic lymphoma kinase [ALK] or c-ros oncogene 1 [ROS-1] translocations are the key biomarkers guiding clinical decision making, although many others are being investigated).
Despite recent breakthroughs, there is still a high unmet need for treatment options in NSCLC. Two relevant studies of the new programmed death receptor-1 (PD-1) inhibitor Opdivo (nivolumab, Bristol-Myers Squibb/Ono Pharmaceuticals) were presented at this year's meeting: CheckMate-017, a phase III trial in squamous NSCLC, and CheckMate-057, a phase III trial in non-squamous NSCLC.
CheckMate-0173 compared Opdivo versus docetaxel as second-line therapy for patients with squamous metastatic NSCLC. This trial randomised 272 patients 1:1 to either treatment with 3 mg/kg of Opdivo every two weeks or 75 mg/m2 of docetaxel every three weeks, both administered until disease progression or unacceptable toxicity.
"Opdivo significantly improved overall survival compared to docetaxel with a 41 per cent reduction in the risk of death"
In this study Opdivo significantly improved overall survival (OS) compared to docetaxel with a 41 per cent reduction in the risk of death, which amounted to a 3.2-month difference at the median (9.2 months vs. 6.0 months). Importantly, the Opdivo survival curve plateaued, with a significantly superior one-year OS rate compared to standard chemotherapy (42 per cent vs. 24 per cent).
In addition, the treatment was generally well tolerated, and higher-grade adverse events (AEs) were manageable.
CheckMate-0574 gave data on Opdivo's performance as second-line therapy in patients with non-squamous metastatic NSCLC. Similar to CheckMate-017, this phase III trial randomised 582 patients 1:1 to treatment with either 3 mg/kg of Opdivo every two weeks or 75 mg/m2 of docetaxel every three weeks, both administered until disease progression or unacceptable toxicity.
In the intent-to-treat (ITT) population, Opdivo significantly improved OS compared to docetaxel. Treatment with Opdivo resulted in a 27 per cent reduction in the risk of death, amounting to a 2.8-month difference at the median (12.2 months vs. 9.4 months). Again, the OS curve plateaued for patients who received Opdivo, resulting in significantly superior 12- and 18-month OS (51 per cent vs. 39 per cent and 40 per cent vs. 20 per cent, respectively).
On the basis of very early data from a single-arm phase II study, Opdivo was approved by the US Food and Drug Administration (FDA) in March 2015 for squamous metastatic NSCLC patients who had previously received a platinum-based therapy, but these phase III results confirm the efficacy seen in that earlier trial and expand its applicability into patients with all histologies.
Dr Tobias Arkenau, a medical oncologist and executive medical director of the Sarah Cannon Research Institute (SCRI) in the UK, calls the data from CheckMate-017 and -057 "a major breakthrough in the treatment of NSCLC and a paradigm shift in how we think about cancer. Historically we treated patients with chemotherapies and suppressed the immune system; now we are activating subsets of immune cells to help recognise cancer proteins. At SCRI we are excited and have been heavily involved in the drug development process of several of those new drugs.
"Encouragingly, these two trials are only the first 'positive' reported trials in NSCLC, and we expect many more positive trials in a variety of cancers, including rare cancer subtypes.
"The data from both trials obviously generate a variety of questions, especially around combinations of immunotherapies with chemotherapy, targeted drugs (EGFR or ALK inhibitors) or other immunotherapies. In addition the duration of treatment required to achieve the best outcome is of interest. In this context several studies are ongoing and I am eagerly awaiting those results over the next few months."
There is also mounting evidence of success for other checkpoint inhibitors in NSCLC: interesting early data for atezolizumab (MPDL3280A, Roche) monotherapy in the POPLAR5 trial and a combination of two immunotherapies pembrolizumab (PD-1-inhibitor) plus ipilimumab (CTLA4-inhibitor) in KEYNOTE-021.6
In the phase II POPLAR trial, the anti-PD-L1 antibody atezolizumab was evaluated against docetaxel in second- and third-line metastatic NSCLC. Interim data with a minimum of 10 months' follow-up in 287 patients who were randomised to receive either 1200 mg of atezolizumab IV every three weeks or 75 mg/m2 of docetaxel every three weeks were reported. The primary endpoint of the trial was OS; at the interim analysis, atezolizumab demonstrated a non-statistically significant 1.9-month gain in OS (11.4 months mOS vs. 9.5 months mOS) in the ITT population.
Despite what may have appeared to be a disappointing OS benefit in the ITT population, the results became more clinically exciting when patients were stratified by their levels of PD-L1 expression (measured as 0, 1, 2 or 3 in either the tumour cells [TC] or tumour-infiltrating immune cells [IC]). Table 1 shows how increasing levels of PD-L1 led to gains in magnitude of progression-free survival (PFS) benefit. Although OS data were not mature for all subpopulations, a similar trend towards greater benefit with increasing level of PD-L1 expression was also seen for OS.
Table 1
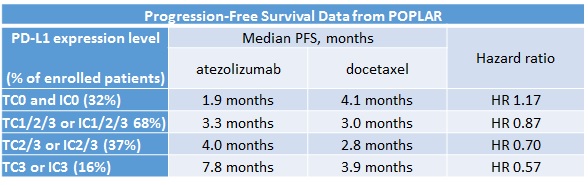
Atezolizumab is currently being tested in a randomised phase III trial (OAK: NCT02008227) in the second-line population that is not limited by PD-L1 biomarker status, similar in design to the CheckMate-017 and -057 trials. This compound is also being explored in several phase III trials in the first-line setting, some of which will restrict enrolment to patients whose tumours express PD-L1.
Very early phase I data of pembrolizumab plus ipilimumab (KEYNOTE-021) as second-line or later therapy were also presented. The primary endpoint of this trial was safety of the combination and the incidence of drug-limiting toxicities (DLTs) during the first three weeks of treatment; secondary endpoints were overall safety and tolerability, overall response rate (ORR) and OS.
A total of 18 patients received four doses of ipilimumab at weeks 0, 3, 6 and 9, with pembrolizumab given every three weeks for up to two years. Four dose levels were planned in the initial protocol, but after emerging data from nivolumab 1 mg/kg + ipilimumab 3 mg/kg every three weeks in NSCLC, the protocol was switched to a single dose level of pembrolizumab 2 mg/kg every three weeks + ipilimumab 3 mg/kg every three weeks.
No DLTs were reported at this dose; however, a substantial rate of Grade 3/4 AEs was observed (17 per cent) that resulted in treatment discontinuation in two patients; no treatment-related deaths were observed. At the time of reporting the ORR was 33 per cent, which is higher than has been observed in prior studies of single-agent pembrolizumab8. The authors stated that the very encouraging results warranted further validation in larger trials.
"One obstacle is how to predict which patients will respond to these new immunotherapies"
One obstacle is how to predict which patients will respond to these new immunotherapies. Several sessions and discussions at ASCO urged the need for new predictive biomarkers, especially in light of conflicting results in assessing the expression status of PD-L1. The PD-L1 biomarker analysis of the above-mentioned trials highlights the hurdles to be overcome in this field.
The POPLAR trial showed that increased levels of PD-L1 expression were correlated with improved PFS and OS benefit. In the CheckMate-057 trial in non-squamous NSCLC, a significant OS benefit was associated with PD-L1 biomarker expression, as shown in Table 2.
Table 2
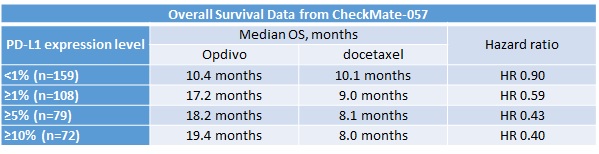
Interestingly, however, in the CheckMate-017 trial, which evaluated Opdivo in squamous NSCLC, all patient cohorts had a significant OS benefit regardless of PD-L1 expression level.
Professor Keith Kerr, a histopathologist from the Aberdeen Royal Infirmary in Scotland, outlined a number of issues that might arise in the real world.
First, all three companies (Bristol-Myers Squibb, Merck Sharp & Dohme and Roche) involved in those studies co-developed their own bioassays with different cut-off rates to measure levels of PD-L1 expression (i.e. 1 per cent, >1 per cent and >5 per cent versus <1 per cent, 1-49 per cent and >50 per cent).
Second, PD-L1 expression was measured on different cells. For example, in the POPLAR trial PD-L1 status was measured in both infiltrating immune cells and tumour cells, whereas in the CheckMate studies PD-L1 expression was measured only on tumour cells.
Prof Kerr also questioned whether, as tumours and their metastases can be very heterogeneous, one or two samples are enough to truly rule out whether someone is PD-L1-positive. This challenge increases when considering that some patient categorisation will be based on archival tissue, and tumours and their biomarker expression can evolve over time.
"One challenge when using these new therapies will be the role PD-L1 expression plays in clinical decision making"
Therefore, one challenge when using these new therapies will be the role PD-L1 expression plays in clinical decision making. Dr Arkenau talked about the complexity of using PD-L1 expression as a biomarker in his practice:
"Not only may the different bio-assays in development or the PD-L1 expression on cancer versus stroma cells affect PD-L1 status, but at this year's ASCO we also learned that PD-L1 expression may differ from tumour type to tumour type. In addition, the expression pattern may vary within tumours; we have seen similar results with HER2 expression, for example, in breast and gastric cancers, where in breast cancer we often see a homogenous and in gastric cancer a more heterogeneous HER2 expression pattern. PD-L1 expression on archival tissue may differ from fresh biopsies. Having said this, I am convinced that the technical/diagnostic challenges can be overcome, and there are clearly early indications that high PD-L1-expressing tumours have better outcomes compared to low or non-expressers.
"In this context we would also need to increase our knowledge of other relevant factors that could predict outcome; interestingly in the recently presented cases of the PD-L1 inhibitor, atezolizumab, and PD-1 inhibitor, pembrolizumab, 'smoking history' was a significant indicator of response to PD-L1/PD-1 inhibition in addition to high PD-L1 expression."
He was excited about the results as they offered a new treatment modality for patients with NSCLC for the first time. "This is important as the immunotherapy side-effect profile overall is generally better tolerated compared to chemotherapy with the risk of infection, neuropathy, hair loss, diarrhoea and the like. Of course it is important to highlight the potential rare immunotherapy-related side effects, especially pneumonitis, colitis, hepatitis and endocrinopathies. As clinicians we need to adapt to a new spectrum of side effects with these drugs and ensure that subtle signs are detected early and counteracted with immune-suppressing agents, such as steroids.
"A rigorous safety management will be even more important when we use double immunotherapy strategies or other immunotherapy combinations, e.g. a checkpoint inhibitor plus a tyrosine kinase inhibitor. This was highlighted in the large phase III CheckMate-0677 study of nivolumab with or without ipilimumab versus ipilimumab alone in patients with metastatic melanoma. Grade 3-4 treatment-related side effects were increased with the combination (nivolumab + ipilimumab 55 per cent versus nivolumab 16 per cent versus ipilimumab 27 per cent) and approximately 30 per cent of patients treated with it had to terminate treatment."
Undoubtedly immunotherapies offer an exciting and potentially paradigm-shifting treatment for patients with cancer. It will be fascinating to see how these drugs evolve the management of cancer in the future.
References:
1 Globocan 2012, available from globocan.iarc.fr; accessed 30 May 2015.
2 Kantar Health, CancerMPact Patient Metrics, available from www.cancermpact.com; accessed 30 May 30 2015.
3 Spigel DR, Reckamp KL, Rizvi NA, et al. A phase III study (CheckMate-017) of nivolumab (NIVO; anti-programmed death-1 [PD-1]) vs docetaxel (DOC) in previously treated advanced or metastatic squamous (SQ) cell non-small cell lung cancer (NSCLC). J Clin Oncol. 2015;33: (suppl; abstr 8009).
4 Paz-Ares L, Horn L, Borghaei H, et al. phase III, randomized trial (CheckMate-057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). J Clin Oncol. 2015;33(suppl; abstr LBA109).
5 Spira A, Park K, Mazieres J, et al. Efficacy, safety and predictive biomarker results from a randomised phase II study comparing atezolizumab (MPDL3280A) vs docetaxel in 2L/3L NSCLC (POPLAR).
6 Patnaik A, Socinski M, Gubens M et al. Phase I study of pembrolizumab plus ipilimumab as a second line therapy for advanced non-small cell lung cancer: KEYNOTE-021 Cohort D.
7 Wolchok J, Vanna S-C, Gonzalez R et al. Efficacy and safety results from a phase III trial of nivolumab (NIVO) alone or combined with ipilimumab (IPI) versus IPI alone in treatment-naive patients (pts) with advanced melanoma (MEL) (CheckMate-067).
8 Garon E, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. NEJM, 2015, 372(21):2018-28.
About the author:
Daniel Blake is UK Oncology Lead at Kantar Health.
Read more from Kantar Health:











