Elon Musk’s Neuralink says FDA has cleared human trial
Neuralink has claimed in a tweet that it has finally received approval from the FDA to start a first-in-human clinical trial of its brain implant chip – but hasn’t indicated when it may start.
The announcement comes just a couple of months after reports emerged that the FDA had turned down Neuralink’s latest request to start testing its Link implant in humans, citing “dozens” of issues that needed to be addressed, including safety issues. So far, the regulator hasn’t commented publicly on the news.
Elon Musk - who owns Neuralink, as well as Tesla, SpaceX, and Twitter - hasn’t commented, other than to send congratulations to the Neuralink team, which has been working on the technology for several years.
Musk has previously said he intends to be one of the first people to have one implanted, but there may be no shortage of willing volunteers, judging by responses to Neuralink’s tweet, even though recipients would have to undergo invasive brain surgery.
“This is the result of incredible work by the Neuralink team in close collaboration with the FDA and represents an important first step that will one day allow our technology to help many people,” said the company.
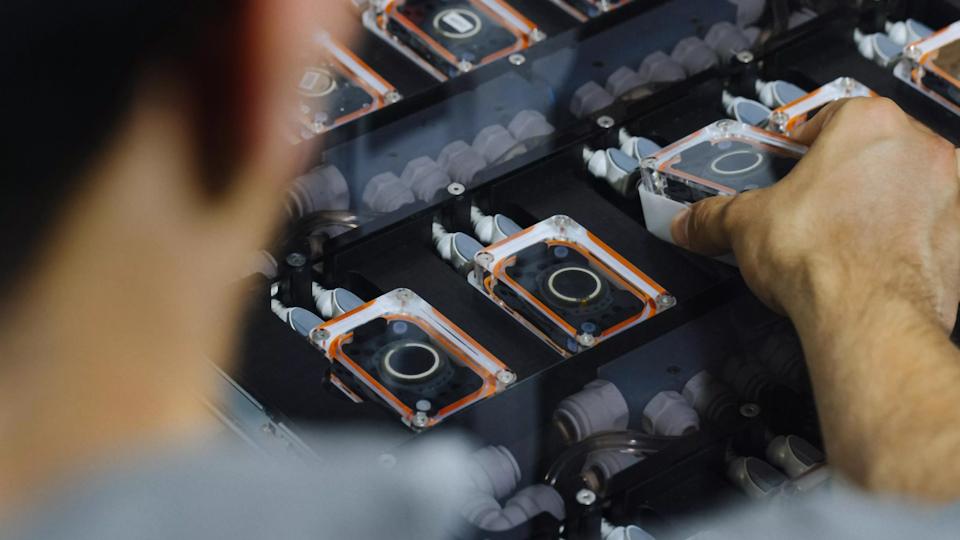
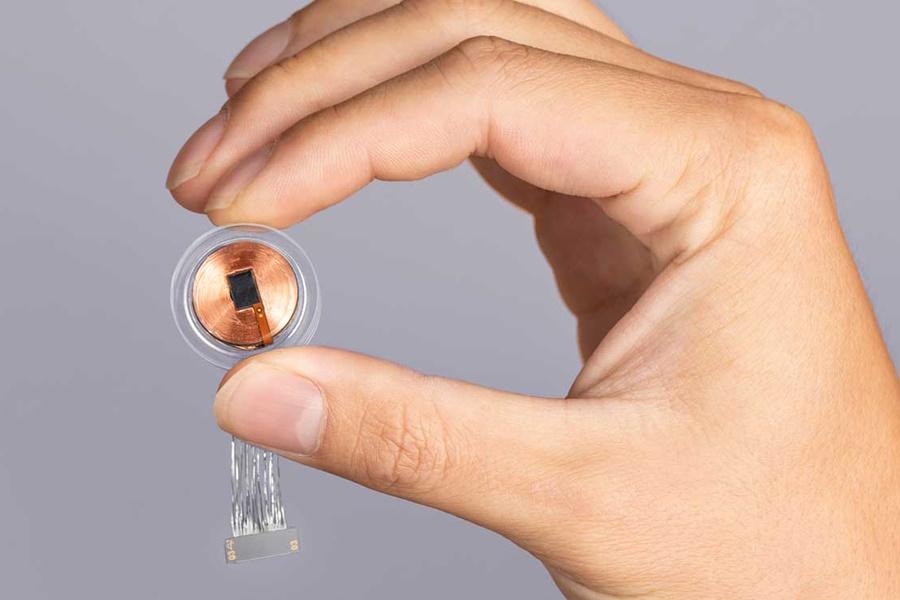
The ‘brain computer interface’ (BCI) device relies on the use of micron-scale threads that are inserted into areas of the brain that control movement or receive sensory information, with each thread containing multiple electrodes that can detect neural signals. The threads are so fine that they have to be implanted using a surgical robot.
Among the proposed applications of the technology is restoration of vision in people who are blind – even if they have been since birth – to restore body functions in cases of where the spinal cord is damaged, and improve on existing deep brain stimulation therapy for Parkinson’s disease.
Near-term, it could be used by paralysed people to control electronic devices using neural signals and Bluetooth technology.
In 2021, Neuralink showcased a pig called Gertrude that had a chip implanted to permit neural activity in her snout to be transmitted and recorded as she rooted for food. The devices have also been implanted into monkeys, allowing them to play the simple computer game Pong or interact with an on-screen keyboard.
If confirmed by the FDA, the approval of a human study will be another inflection point for the embryonic implantable BCI sector, but won’t be the first for that sector. Blackrock Neurotech has already tested its BCI in humans, while another player – called Paradromics – has said it plans to start trials of its Connexus Direct Data Interface in 2024.
There are also a clutch of companies developing non-implantable BCIs, where the electrodes are placed around the scalp, but they may lack the accuracy of those embedded within the brain.