FDA said no to trials of Elon Musk’s Neuralink chip; report
Elon Musk’s hope of soon starting clinical trials of its Neuralink wireless brain chip have been dashed by the FDA, at least for now.
That is according to a Reuters report, which said the US regulator had identified “dozens” of issues that Neuralink will have to address before it will grant approval for the device to be trialled in humans.
Musk has said on a number of occasions that clinical trials of the brain implant interface – designed to connect humans and machines directly – have been poised to start trials since 2019, but an application to do so was reportedly only filed with the FDA last year.
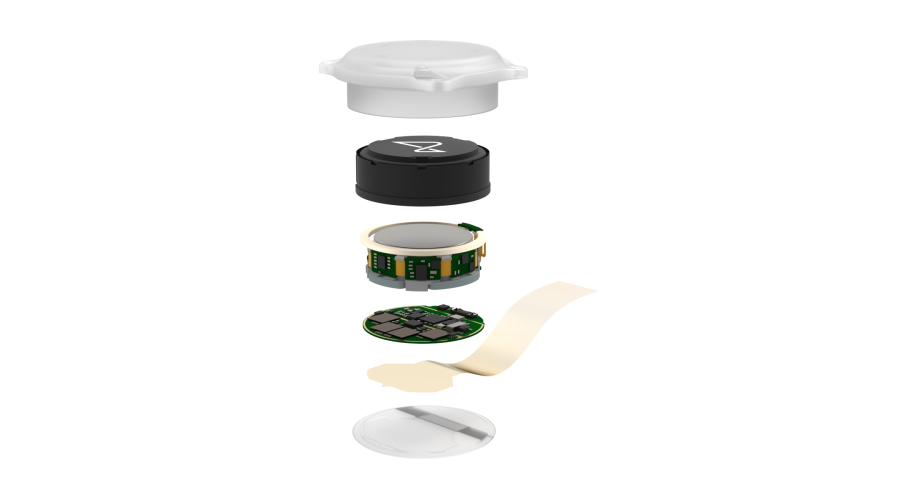
Among the concerns raised by the FDA are the safety of the lithium battery used to power the device, the potential for the micron-scale ‘threads’ that are inserted into areas of the brain to migrate elsewhere, and whether it can be removed without damaging brain tissue, said Reuters, which said the information came from “current and former” employees of the company.
Among the potential applications of the technology is restoration of vision in people who are blind – even if they have been since birth – and to restore body functions in cases where the spinal cord is damaged, said Musk at an event towards the end of last year.
Musk has not responded to the report on Twitter, despite prolific Tweeting since he acquired the social media platform for $44 billion last year.
In 2021, Neuralink showcased a pig called Gertrude, which had a chip implanted that allowed neural activity in her snout to be transmitted and recorded as she rooted for food. The devices have also been implanted into monkeys, allowing them to play the simple computer game Pong or interact with an on-screen keyboard.
Earlier this year, the US Department of Transportation launched an investigation into Neuralink over the potential movement of hazardous pathogens in tissue samples and implants removed from the brains of monkeys. The company has insisted it is in “full compliance” with federal regulations.
It has also been forced to defend itself from allegations of animal welfare violations, including those levelled by the Physician’s Committee for Responsible Medicine, an animal-welfare advocacy group, which is calling for the company to release data from experiments on monkeys, including side effects from the procedure, such as infections. A PCRM complaint also triggered the transport department probe.