Is there a faster way to build clinical trials?
As you know, clinical trials can be complex. Industry standards are now required for clinical trial submission. This means even more associated costs, resources, time, and effort. The Formedix ryze clinical metadata repository (MDR) can help!
So what can it do for you? In a nutshell, it can help you:
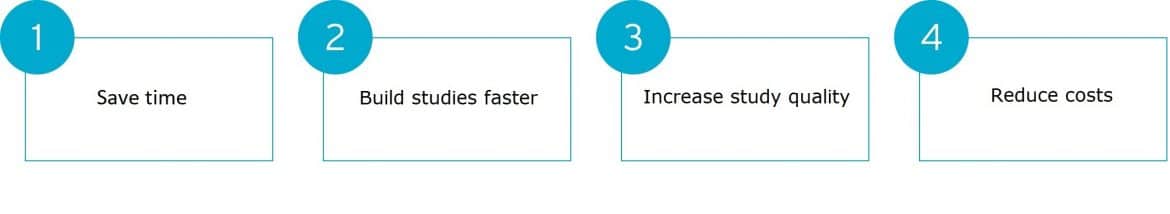
How is this possible?
Take studies and standards for example. Ideally, you want to manage them all in one place. Right? The Formedix clinical metadata repository lets you do just that. It’s really a fancy web platform for managing your clinical trial metadata.
Import your organizational standards straight from your database into the clinical metadata repository. Then you’re ready to go! Already you’ve saved a huge amount of time getting started.
Plus, now you won’t spend time searching all over for files and spreadsheets. You can easily search across all the content in your MDR. And you and your teams can work together like never before. No information gaps and no duplicated activities. You’ve got full transparency.
You can define your own role-based access if you want. What content you want to lock down and what you want to make available. You can set up different types of access to studies and standards. Even to different individuals and teams. Whatever works best for your company!
Dealing with change is often a real headache. And if it’s not done correctly, you risk regulatory non-compliance. So Formedix lets you create and manage change requests really easily. If one person makes a change, the content is locked down. No conflicts. And full traceability makes it easy to see what’s been changed.
Impact analysis is even built-in. This lets you see the impact of any changes. And you can easily identify any content that needs to be updated in response to a change.
Want to build studies faster?
Well, once they’re in ryze clinical metadata repository, you can reuse your standards and studies as often as you like. That saves you retyping them each time.
And because your content is standardized – in other words, it’s already been approved – you know it’s correct. This helps you keep data nice and consistent, and maintains compliance across studies. That’s a big tick in the quality box, as well as saving even more time.
What about EDC build?
Formedix lets you build, preview, and validate studies for most EDC systems. That’s the leading 7 including Rave and InForm. |
The best thing is, you can preview exactly what your CRFs and annotations will look like in your EDC – before you’ve built your study! When you’re ready to export, you can be confident it’s going to be as expected. You can read more about CRFs in Everything you want to know about CRFs and Why switch to automated CRF annotations?.
Using Rave EDC?
Formedix fully integrates with Rave. That means you can import and export your studies directly to and from Rave! Again this saves you a huge amount of time. A study that typically takes 21 weeks to build can take as little as 14 weeks with Formedix. That’s the kind of time and resource-saving you’re looking at.
Want to make things even easier?
Our API lets your internal system automatically communicate with the Formedix platform. For example, you could automatically upload/download datasets between Formedix and your in-house database. How efficient is that!
So your clinical trial design, build, and submission can be so much faster than before. The time and resources you save mean potential cost savings too. Sound too good to be true? We can show you the Formedix metadata repository and related tools. Just request a demo.
The good thing is, our MDR is off the shelf too. So you can be up and running straight away. You don’t need to wait for us to build you it from scratch. But likewise, if you need any customizations, our in-house development team can make it happen.
If you’ve found this post interesting, you might be interested in Everything you want to know about CRFs and Can clinical study build be a piece of cake?
And if you need a helping hand along the way?
No problem. Our Professional Services team is here to support you. Think of them as part of your wider team. We understand a lot of the daily pressures and difficulties you go through. After all, we’ve been in the business for 20 years. That’s a lot of time spent getting to know what you’re up against. And that’s how we’re able to help, with solutions that target and solve those chronic pain points!
It’s not just like that at the start. We keep listening to what you need and we’re constantly improving our platform. In fact, we use your feedback to help us do this.
Heard enough yet? Are you ready to see how Formedix can help you specifically?
Great. Now set aside some time to tell us all about you and your organization. We need to understand what makes you tick… and what gives you the most grief! Call us now and get a date in the diary. Remember, it’s just an initial discussion. There’s no obligation, so you’ve got nothing to lose!













