Rethinking access barriers to innovation
Market access issues don’t stop once a product has passed HTA – and this is especially true for disruptive therapies like ATMPs. Experts from Executive Insight discuss how a holistic, multi-stakeholder approach can help companies overcome access barriers at all stages.
This article appears in our digital magazine Deep Dive: Market Access 2021. Read on for a preview:
The last decade has seen some remarkable advances in medicine, with innovative new products like cell and gene therapies showing efficacy in diseases long thought almost untreatable.
But launching a disruptive product can be a double-edged sword. While the potential patient benefits are huge, these therapies face difficulties in passing health technology assessment (HTA) processes designed for more standard products, and once approved they face other barriers from putting new pressures on health systems.
Michalina Jenkins, who has assessed HTA systems in terms of associated barriers in a number of different countries in her role as a senior consultant at Executive Insight, says that one of the most common HTA barriers companies face is a lack of broad value recognition for innovative products.
“When considering the value of innovation, we know that it should drive benefits to patients – but also, broadly speaking, it should provide cost savings to the healthcare system and improve the wellbeing of society in general,” she says.
In the post-launch environment, the effects of not having a broad recognition of value become even sharper, says Luca Lorenzi, manager at Executive Insight.
He notes that, by their nature, these innovative products will often enter an environment that is not ready to support their access and adoption.
“Most importantly, there might be a lack of sufficient funding mechanisms available at launch – and if there is a high burden for healthcare professionals to obtain funding, that will be a major hurdle for adoption of the technology.
“It’s important to look at the environment not only from a product angle, but also from an associated services angle – e.g. will the technology cause extra costs because of new procedure, diagnostics or administrative needs that might not receive sufficient funding at launch?”
Additionally, companies may find that there are no optimal care pathways to allow optimal integration of the innovation into the healthcare system.
“This all comes down to whether the existing care pathways or process infrastructures are fit for purpose for your innovation, and whether your innovation has a specific complexity in the way it’s delivered,” says Lorenzi.
“This includes factors like the infrastructure and capacity of treatment sites. We saw with CAR-Ts, for example, that some healthcare systems intentionally limited the number of sites that could deliver this technology to better control its usage.”
Similarly, a particularly disruptive product may require behaviour change from HCPs and patients, which can require additional time and cost investment.
Additionally, companies may find that there are no optimal care pathways to allow optimal integration of the innovation into the healthcare system.
“This all comes down to whether the existing care pathways or process infrastructures are fit for purpose for your innovation, and whether your innovation has a specific complexity in the way it’s delivered,” says Lorenzi.
“This includes factors like the infrastructure and capacity of treatment sites. We saw with CAR-Ts, for example, that some healthcare systems intentionally limited the number of sites that could deliver this technology to better control its usage.”
With such a wide range of factors to consider across the entire access landscape, pharma needs to start planning for potential barriers as early as possible in development – and that means working with key stakeholders systematically and repeatedly to shape the environment before launch.
Companies need to sit down with a broad group of stakeholders who share common needs and also want to be actors in this change.
All parties can then work together to identify and prioritise key barriers at all levels, and shape the access environment into that ideal position.
“That means you need to identify those stakeholders that have influence over shaping the system, and that may differ from country to country,” says Jenkins.
Lorenzi adds that it’s important to apply the patient perspective throughout all of these discussions.
“A clear advantage of doing that is it allows you to engage stakeholders more effectively. If you assume a patient perspective rather than a product perspective, you are talking to them in their language. That means attempts to shape the environment will be more successful and have more visibility.”
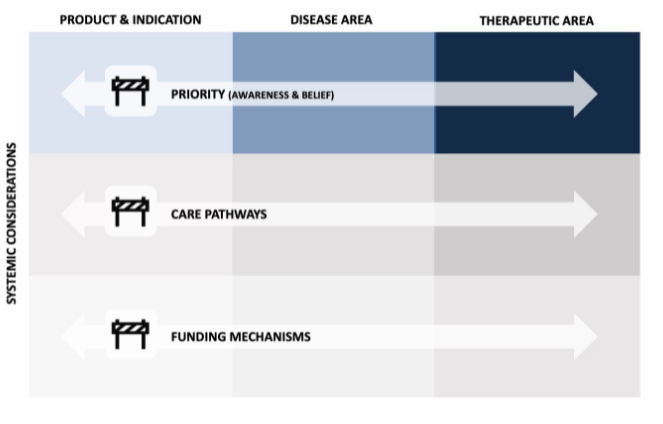
It’s also important to consider barriers not just at a product level but also at an indication and disease area level.
For example, if you have a product in diffuse large B-cell lymphoma (DLBCL) you might want to also look at access issues in lymphoma more generally.
You essentially need to take a three-by-three matrix view. That means considering disease perceived priority, care pathways, and funding mechanisms, and looking at those three buckets at the product, indication, and disease and therapeutic area level.
• Read the full article in pharmaphorum's Deep Dive digital magazine












