Alimetry nabs FDA okay for gastric disorder-diagnosing wearable
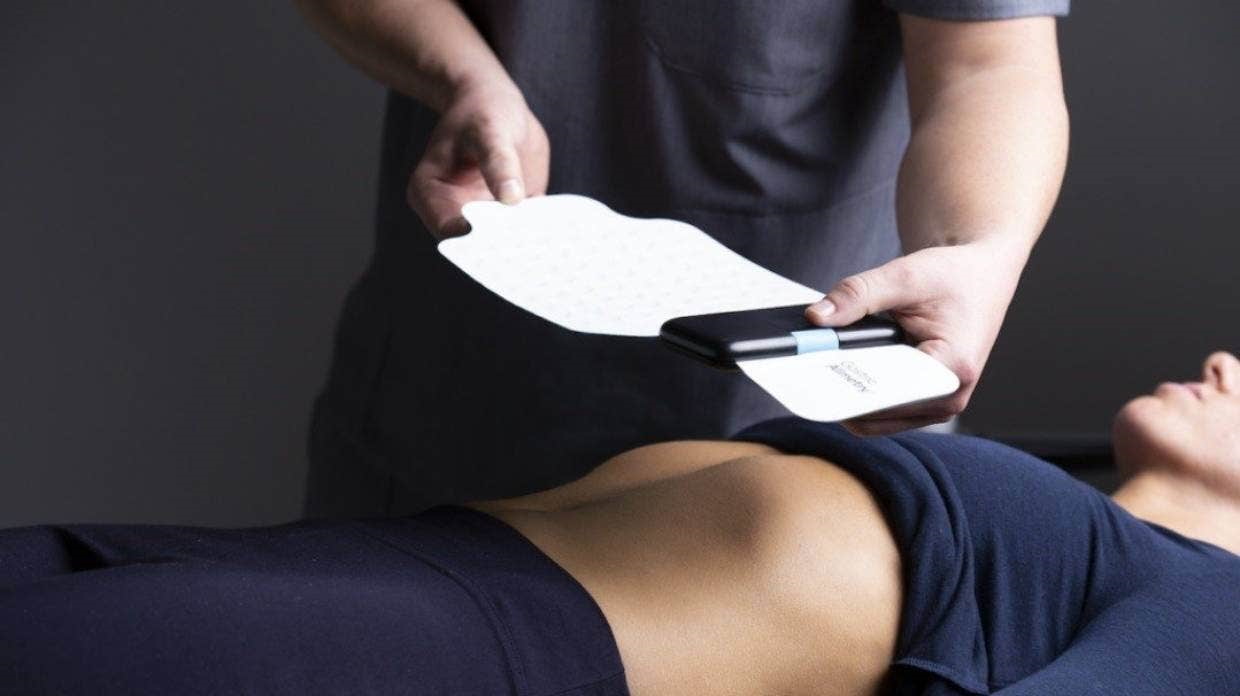
New Zealand medical tech startup Alimetry has claimed an FDA approval for a wearable device that can be used to diagnose gastric disorders non-invasively.
The Gastric Alimetry device will be marketed and distributed in the US by a new Minneapolis-based subsidiary, said the company, which has also secured approval for the product in New Zealand as well as the UK.
The device (pictured above) – Alimetry's first commercial product – uses a stretchable high-resolution sensor to non-invasively map digestive patterns by measuring electrical signals through the skin, and delivers clinical reports via the cloud to inform the diagnosis and classification of gastric disorders.
Gastric Alimetry is due to be launched in the US next month, with the help of a recent fundraising at the Auckland company which raised NZ$16 million (around $10 million). It has also had a limited rollout in New Zealand and the UK.
The testing is performed in a clinical setting, rather than at home, with recordings from the skin surface taken before and after a meal while patients log symptoms into a companion app.
"Diagnosing gastric symptoms has been a deeply challenging clinical problem," said Alimetry's chief executive and founder Greg O'Grady, a professor of surgery at the University of Auckland.
"Existing tests are frequently unreliable and inconclusive, and patients may undergo months or even years of testing – often costly, invasive, or involving radiation – only to end in confusion and trial-and-error care," he added.
" Gastric Alimetry is a game-changing tool that will bring improved clarity to field, enabling enhanced clinical outcomes, and safer, more accessible, and less-invasive care."
Gastric disorders – such as nausea and vomiting, gastroparesis, and functional dyspepsia – affect more than 8% of the world's population, according to paper published last year in the journal Gastroenterology.
Functional dyspepsia alone was estimated to cost the US upwards of $18 billion in healthcare expenses, according to figures published in 2013.
O'Grady is also chief scientific officer at The Insides Company, which has developed a device for reinfusing gastric fluid that passes into the small intestine (chyme) back into the stomach, helping maintain the nutritional status of people with acute intestinal failure.
Alimetry was spun-out of the University of Auckland in 2019.











