Artificial pancreas outperforms current tech in type 1 diabetes
An “artificial pancreas” has proved to be more effective at controlling blood sugar than injections with insulin by patients or with a pump, in an international trial mainly funded by the US National Institutes of Health.
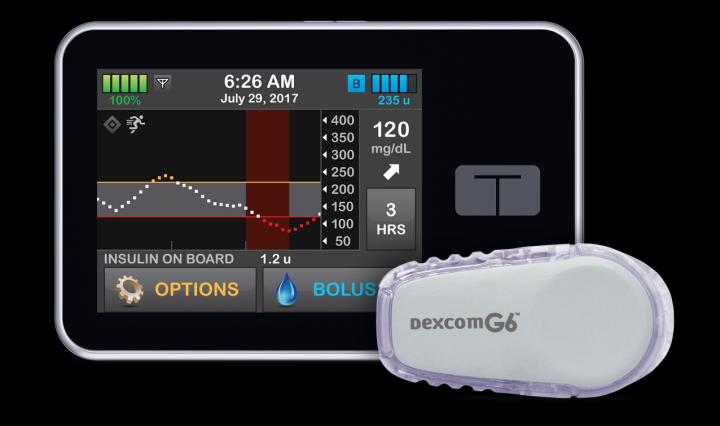
The artificial pancreas, also known as a closed-loop control, is an “all-in-one” diabetes management system using a continuous glucose monitor (CGM) and automatically delivers insulin when needed using a pump.
Importantly, the system used in the trial includes several safety features, such as a safety module dedicated to preventing hypoglycaemia, and a system where control is gradually intensified overnight to achieve near-normal blood sugar levels every morning.
This is one of four NIH-funded research efforts testing and refining artificial pancreas technology, looking at safety, efficacy, user-friendliness, physical and emotional health of participants, and cost.
It replaces reliance on testing by fingerstick or CGM with separate delivery of insulin by multiple daily injections or a pump.
The International Diabetes Closed-Loop (iDCL) Study involves five separate artificial pancreas clinical protocols implemented by 10 research centres in the US and Europe.
This six-month study was the third phase in a series of trials and was conducted with participants living their usual day-to-day lives, so researchers could best understand how the system works in typical daily routines.
This iDCL protocol enrolled 168 participants aged 14 or older with type 1 diabetes. They were randomly assigned to use either the artificial pancreas system called Control-IQ or sensor-augmented pump (SAP) therapy with a CGM and insulin pump that did not automatically adjust insulin throughout the day.
Participants had contact with study staff every two to four weeks to download and review device data. No remote monitoring of the systems was done, so that the study would reflect real-world use.
The study found that using the artificial pancreas system significantly increased the amount of time spent with blood sugar in the target range of 70 to 180 mg/dL by an average of 2.6 hours a day since beginning the trial.
The time spent in range in the control group remained unchanged over six months and the artificial pancreas users also showed improvements in time spent with high and low blood glucose, haemoglobin Alc, and other measurements related to diabetes control compared with the control group.
There was high adherence in both groups and 100% participant retention, and no severe hypoglycaemia events occurred in either group.
Diabetic ketoacidosis occurred in one participant in the artificial pancreas group due to a problem with equipment that delivers insulin from the pump.
Control-IQ was derived from a system originally developed at the University of Virginia, by a team led by Boris Kovatchev, director of the UVA Center for Diabetes Technology with funding support from the National Institute of Diabetes and Kidney Diseases (NIDDK).












