Litmus Health launches to help improve clinical trial efficiency
Litmus Health, developer of a machine learning platform to improve phase 1 and 2 trial efficiency, has entered public beta.
The platform, which takes data from over 200 data sources including surveys and wearables, uses machine learning algorithms to build correlations between behaviour, environment and patient outcomes, resulting in better trial management, better-informed trial endpoints, and better quality of life for participants.
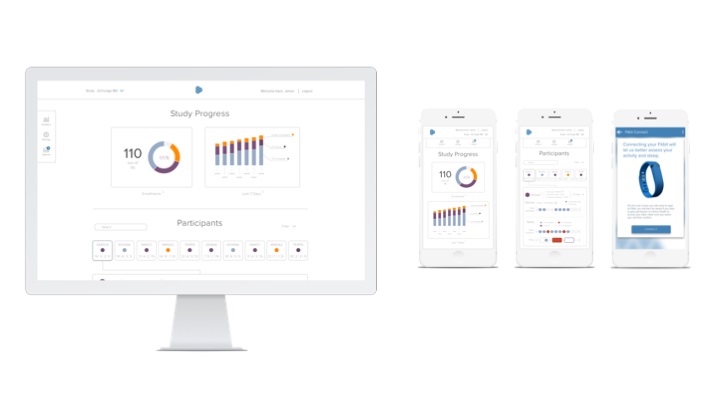
The gathered data is displayed in a dashboard format, allowing researchers to view individual participants’ data.
The Litmus platform will first be piloted in a trial funded by Takeda Pharmaceuticals and conducted by the University of Chicago studying the effects of activity, sleep and diet on inflammatory bowel disease (IBD).
The trial will begin with a 100-person cohort with the intent to expand to 600. Data will be gathered using Fitbits.
“The answers we need are everywhere around us,” said Dr. Samuel Volchenboum, chief science officer of Litmus, and director of the Center for Research Informatics at the University of Chicago. “We need a better way to collect data in clinical research. Smartphones, wearables, and home sensors present a unique opportunity. Most researchers understand the value of patient-generated information collected at the point of experience, but they have no good way to harness those data. The ability to measure outcomes in multiple dimensions, remotely, is key.”
“These devices are already in the hands of consumers,” added Daphne Kis, CEO of Litmus. “The challenge is to credibly accommodate the data they collect. We have the opportunity to help researchers understand patients and their quality of life as we never have before, and the market is ready. These data are going to have huge implications for the healthcare ecosystem and for how we use patient data both in the clinical trials setting and beyond.”
Unlike the majority of machine learning solutions currently available, Litmus specifically focuses on phase 1 and 2 clinical trials. The intention is to address one of pharma’s biggest problems: getting drug candidates to market.
Currently, only a small percentage of drug candidates successfully make it from phase 1 through to phase 4 trials at which point candidates need to make it through strict regulations.
"The business proposition here is that pharma’s broken in some ways," CEO Daphne Kis told MobiHealthNews. "The clinical trial process is analogous to the VC world where one in 10 or 15 hits pays for all the other failures. That as a long-term model is not really a business model that works. What we are seeing is, part of the interest from pharma is that tools like ours that are data-driven provide pharma with help in making drug development decisions. We’re specifically focusing on phase 1 and 2 before the hockey stick of phases 3 and 4 in the hopes that it will have an impact in the business decision of whether to go ahead with drug development, based on having better, more correlated data to make those decisions."